The daily routines of life are tied to the patterns of the environment, resulting in the evolution of circadian rhythms. Cues such as temperature, sunlight, food, and sound, called zeitgebers, adapt circadian rhythms to external conditions. Growing evidence links circadian disruptors or zeitgebers to adverse outcomes in humans.
A comprehensive overview of the relationship between metabolic health and circadian gene expression is lacking. As such, the present study summarized and compared evidence from animal models with findings from epidemiological research to advance the understanding of the contribution of circadian disruption and clock gene expression to metabolic health-related pathologies.
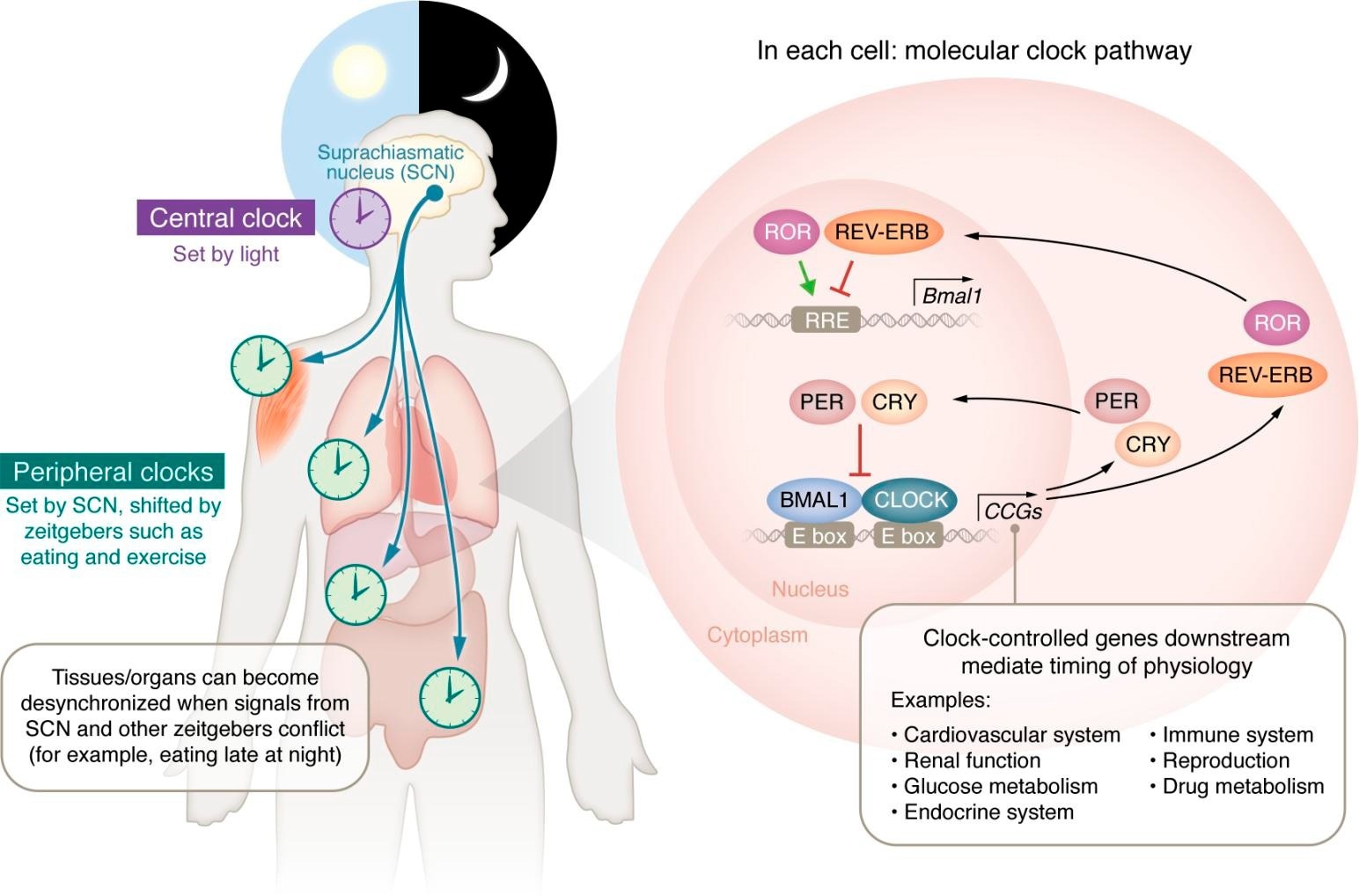 Circadian control of molecular core clock gene signaling and physiologic regulation. The central, peripheral, and molecular clocks and the physiological processes under circadian control. The circadian clock (purple) in the suprachiasmatic nucleus (SCN) of the brain sets peripheral clocks in individual organs and tissue types (light green) via signals including circulating hormones, metabolites, the sympathetic nervous system, and body temperature. Within the cells of the SCN and each organ/tissue type, each cell contains transcription-translation feedback loops, the molecular clocks that drive circadian rhythms. These molecular clocks regulate the transcription of thousands of CCGs and direct the daily oscillatory expression of thousands of COGs and additional transcription factors that mediate the timing of myriad physiological processes as represented in the molecular clock pathway within cells. Study: Circadian disruption, clock genes, and metabolic health.
Circadian control of molecular core clock gene signaling and physiologic regulation. The central, peripheral, and molecular clocks and the physiological processes under circadian control. The circadian clock (purple) in the suprachiasmatic nucleus (SCN) of the brain sets peripheral clocks in individual organs and tissue types (light green) via signals including circulating hormones, metabolites, the sympathetic nervous system, and body temperature. Within the cells of the SCN and each organ/tissue type, each cell contains transcription-translation feedback loops, the molecular clocks that drive circadian rhythms. These molecular clocks regulate the transcription of thousands of CCGs and direct the daily oscillatory expression of thousands of COGs and additional transcription factors that mediate the timing of myriad physiological processes as represented in the molecular clock pathway within cells. Study: Circadian disruption, clock genes, and metabolic health.
Circadian rhythms in animal models
Genetic drivers of circadian rhythms in animals were first discovered in the fruit fly, Drosophila melanogaster, which revealed that the period gene (per) and protein (PER) were essential to the circadian clock. Further studies corroborated these findings and also discovered additional core clock genes (CCGs), brain and muscle ARNT-like 1 (BMAL1), cryptochrome (CRY), and PER orthologs (PER1 – PER3).
In a Clock gene-mutant mouse model, animals showed altered timings of food intake with ad libitum feeding and consumed more calories beyond the active phase; they developed metabolic syndrome and obesity and dampened activity rhythms. Similar metabolic changes were noted in other mouse models with mutations in molecular clock components.
Glucose homeostasis is affected by cell-specific gene signaling mechanisms regulated by specific CCGs. A study found that, in mouse pancreatic β cells with intact BMAL1 expression, BMAL1/CLOCK dimers were bound to regulatory sites, i.e., CCGs, driving transcription of targets (clock output genes), distinct from cells in the liver. By contrast, those with disrupted BMAL1 developed glucose intolerance.
Most BMAL1/CLOCK binding sites in β cells are not identified in other tissues, supporting a tissue-specific role of the clock in metabolic health. Substantial efforts have been invested in studying the effects of circadian disruptors in mice, including altered activity, sleep, light exposure, and food intake. Studies mimicking shift-work exposures have reported that circadian disruptors could alter CCG expression and metabolic health.
Further, wild-type mice with misaligned food intake timing relative to inactive and active periods exhibited accelerated weight gain. Mice that consumed a high-fat diet in inactive periods had higher weight gain and body fat percentage than those fed the same in their active period. Contrastingly, mice fed a high-fat diet with time-restricted feeding were protected from weight gain.
Evidence from epidemiological and population-based research
Preliminary evidence suggests that metabolic health is altered due to circadian disruption from epidemiological analyses of shift workers. The Nurses’ Health study noted that subjects exposed to night-shift work had higher calorie intake, shorter sleep durations, and increased odds of obesity. One study found higher postprandial ghrelin levels and lower xenin (a gut-derived hormone) in the bloodstream in night-shift workers.
Further, 21 adults were subjected to circadian disruption (sleep restriction). Three weeks later, circadian-disrupted adults had significantly reduced resting metabolic rates and higher fasting/postprandial glucose levels compared to baseline; these changes normalized nine days after returning to standard wake/sleep patterns.
CCG expression and circadian disruption
A study involving 18 nurses noted fewer rhythmic genes in peripheral blood mononuclear cells of rotational night shift workers than day shift workers. Another study involving 60 nurses revealed differential expression of almost all CCGs. In a separate study, 22 individuals were subject to forced desynchrony of 28-hour days, and sleep onset was pushed four hours each night.
While the 24-hour melatonin rhythms were largely preserved, circadian transcripts had up to a six-fold reduction. Further, thousands of transcripts had altered expression in a study with 26 sleep-restricted individuals, with PER1, PER2, PER3, CLOCK, and CRY2 being significantly affected. Moreover, multiple metabolic and oxidative stress genes were also altered upon sleep restriction.
Metabolic pathology and CCGs
There may be a bidirectional relationship between CCG expression and metabolic health, as worsened metabolic health, per se, can alter CCG expression in a tissue-specific manner. A study with 28 morbidly obese and 21 lean female non-shift workers without diabetes revealed altered expression of various CCGs in obese subjects. Further evidence indicates that weight loss can alter CCG expression.
Moreover, studies have suggested that even short-term exposure to circadian disruptors could alter CCG expression and metabolic pathways. One study of 14 healthy males allowed three days of normal sleep and reversed the day-night schedules for the next three days. Free fatty acids and fasting glucose levels were significantly higher upon disruption than during normal conditions.
Concluding remarks
A growing body of evidence links clock activities to pathological metabolic outcomes. From a risk paradigm, the impact of circadian disruptors depends on the exposure duration and quality. An example would be alternating shift work. In a tissue desynchrony model, the metabolic profiles of tissues are misaligned due to differential response times needed to re-establish normal rhythms following inappropriate zeitgeber exposure or circadian disruption.
Prolonged periods of alternating shift work may not allow internal organ re-entrainment. Further, shift work is not always synonymous with circadian desynchrony, as there could be shift workers with appropriate circadian hygiene. Thus, uncovering which components of circadian misalignment contribute to poor health is complex. Overall, further research is needed to expand the evidence base and advance the understanding of these relationships.
Journal reference:
- Schrader LA, Ronnekleiv-Kelly SM, Hogenesch JB, Bradfield CA, Malecki KMC. Circadian disruption, clock genes, and metabolic health. Journal of Clinical Investigation, 2024, DOI: 10.1172/JCI170998, https://www.jci.org/articles/view/170998