Microbiota is a crucial determinant of human health and disease. The human gastrointestinal (GI) tract hosts the majority of the microbiota, which co-evolves with the host organism. Evidence suggests that a bidirectional communication network, i.e., the GBA, exists between the central nervous system (CNS) and the gut microbiota.
Studies suggest that the gut microbiota plays a role in neurodevelopment, neuroinflammation, emotion and behavior regulation, and cognitive processes. As such, the gut microbiota is a multi-functional target with immense potential in treating neurological disorders. In the present study, researchers reviewed the role of GBA in neurological disorders.
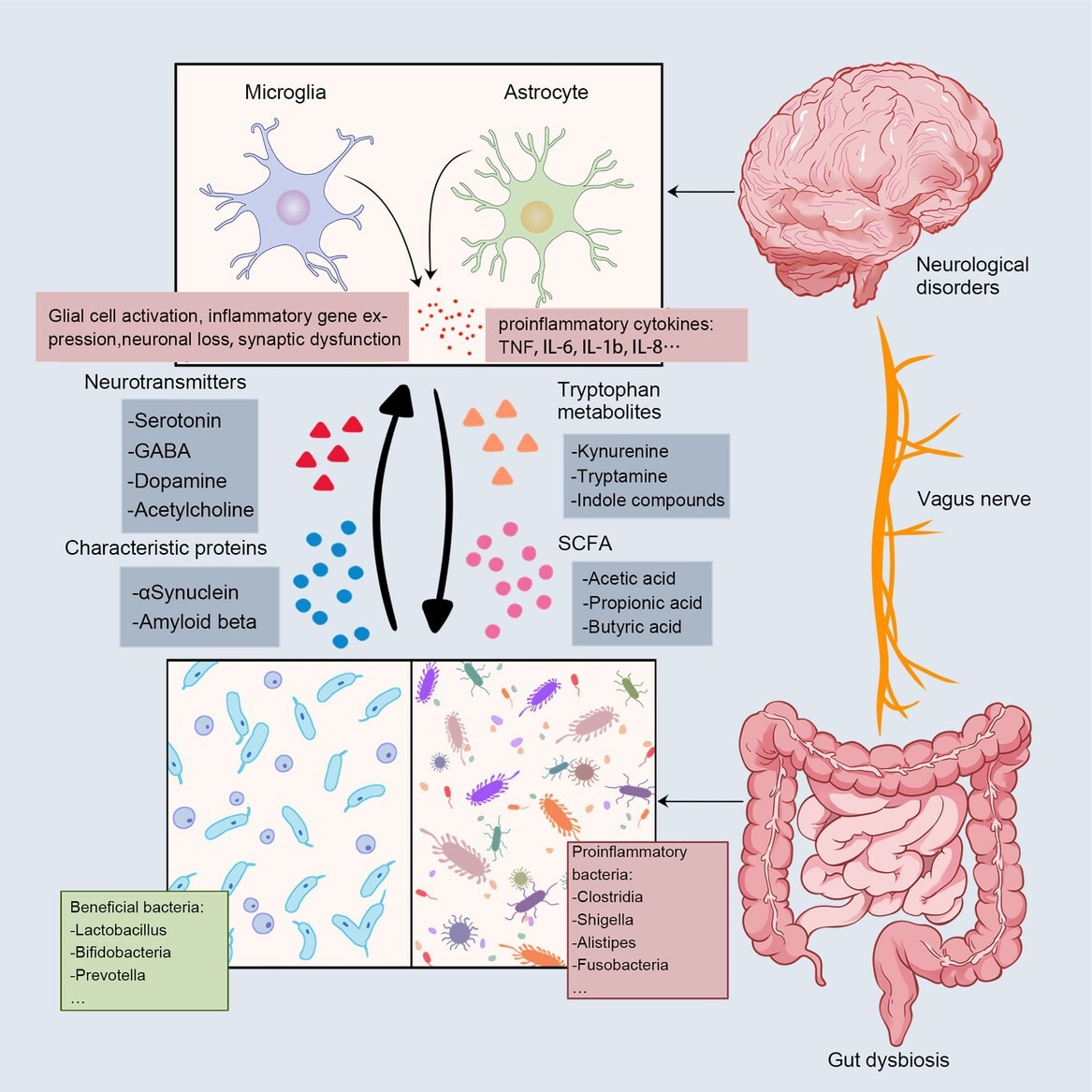
Bidirectional communication between the gut and the brain. The main communication pathways between microbes and the brain include neural pathways, immune pathways, and metabolic signals. Gut disorders send signals to the brain via the vagus nerve, and a decrease in beneficial bacteria and an increase in proinflammatory bacteria cause altered levels of microbial metabolites, including neurotransmitters, SCFA, and indole metabolites. The deposition of characteristic proteins in neurodegenerative diseases has also been associated with gut microbiota (Aβ in AD; αSyn in PD). These signals stimulate glial cells in the brain, the expression of proinflammatory genes, neuronal loss, synaptic dysfunction, and the rise of proinflammatory cytokines. Review: The gut microbiota–brain axis in neurological disorders
GBA and communication mechanisms
The GBA maintains the homeostasis of the CNS, GI tract, and microbial system through multiple pathways. Studies in mouse models and germ-free mice treated with antibiotics have demonstrated the effects of microbial signaling inhibition on neurodevelopment and induction of neurological disorders. Further, the lack of gut microbiota is associated with higher blood-brain barrier permeability.
Besides, wild-type mice transplanted with microbiota from mice with Alzheimer's disease (AD) developed memory impairments and colonic inflammation. Likewise, transplanting microbiota from healthy mice to those with Parkinson's disease (PD) alleviated neuronal damage and neuroinflammation. Major depression, psychosis, bipolar disorder, and schizophrenia cause gut dysbiosis.
Nevertheless, probiotics and prebiotics can boost levels of specific microbes, with reports suggesting that probiotics can be effective in preventing ailments. The gut and the brain communicate through the vagus nerve (VN) and the autonomic nervous system. VN can sense and transmit microbiome information to the CNS. It also mediates gut bacterial effects on the brain. The enteric nervous system (ENS), regarded as the second brain, comprises a network of glial cells and neurons.
The ENS regulates the GI tract's motility and secretory functions. The gut microbiota partly induces its function and development. Gut microbiota depletion leads to neuronal loss, reduced gut glial cells, and increased intestinal permeability. However, a spontaneous recovery of the gut microbiota restores the enteric neuronal loss and impaired GI physiology.
GBA and neurological disorders
Autism spectrum disorder (ASD) is characterized by restrictive, repetitive behavior and impaired social communication. Its etiology is unknown; however, ASD is likely associated with the gut microbiota. ASD patients exhibit a characteristic gut dysbiosis relative to healthy controls and have increased microglia density and greater distance between neurons and microglia.
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, impaired impulsivity, motor hyperactivity, and inappropriate development. Studies have revealed differences in the gut microbiota composition between healthy individuals and ADHD patients. AD, a neurogenerative disease, is the most common cause of dementia and is characterized by impaired memory and cognition.
Fecal microbiota transplantation (FMT) from AD mice induces neuroinflammation and memory impairment in wild-type counterparts. Besides, long-term FMT from healthy mice can alleviate tau pathology, amyloid-β (Aβ) deposition, and memory impairments in AD mice. AD patients exhibit substantial differences in the relative abundance and composition of the gut microbiota. Depression is a common mental health problem. Gut microbiota is among the factors influencing depression.
Individuals with depression have a higher prevalence of Alistipes and Enterobacteriaceae and reduced Turicibacteraceae. Anxiety is another prevalent mental health issue. Depressive patients often experience anxiety. Specific bacterial taxa, such as Bacteroidetes, Sutterella, Faecalibacterium, Lactobacillus, are associated with anxiety and depression.
Gut microbiota modulation as a therapeutic intervention
Bifidobacterium and Lactobacillus are the most studied probiotics for neurological disorders, and their use has been associated with improvements in patients. Galacto- (GOS) and fructo-oligosaccharides (FOS) are dietary prebiotics with human health benefits. GOS and FOS can suppress interleukin-1β and reduce inflammation-related anxiety. Besides, GOS administration in ASD children increased their social behavior scores.
FMT also stands out as a promising therapeutic intervention for neurological disorders. It can ameliorate stress-induced intestinal inflammation, gut dysbiosis, depression-like behavior, neuroinflammation, and intestinal mucosal destruction in rats. FMT has been shown to improve motor deficits, reshaping the gut microbiota in PD patients. It also augmented behavior symptoms in ASD patients. Diet has a profound impact on the gut microbiota.
Diets enriched in plant-based foods, antioxidants, and fiber, with low fat and meat, are associated with lower risk of PD, AD, stroke, and migraine. By contrast, diets involving high-fat items, processed foods, red meat, and refined carbohydrates are linked with elevated inflammation and risk of neurological disorders.
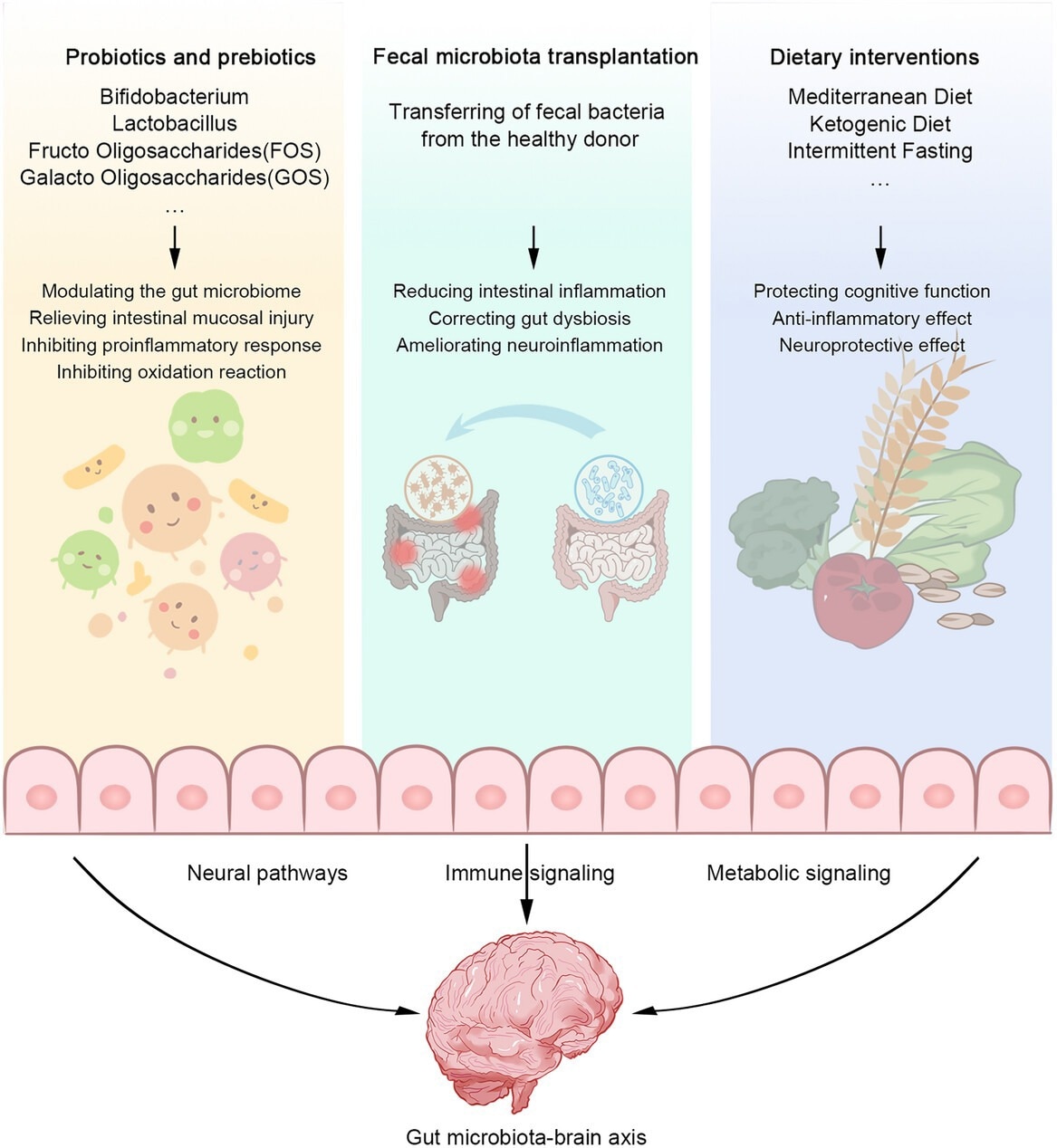
Therapeutic interventions to modulate the gut microbiota. Supplementation with probiotics and prebiotics (e.g., Bifidobacterium, Lactobacillus, FOS, GOS) regulates gut microbiota, reduces intestinal mucosal injury, inhibits proinflammatory response, and inhibits oxidative reaction. FMT reduces intestinal inflammation, corrects gut disorder, and ameliorates neuroinflammation. Dietary interventions (Mediterranean diet, ketogenic diet, and intermittent fasting) can protective cognitive function, anti-inflammatory effects, and neuroprotective effects. These therapeutic interventions act on the gut–brain axis through neural, immune, and metabolic signaling pathways to improve gut health and, in turn, neurological disorders progression.
Concluding remarks
Taken together, the GBA regulates the host's health via multiple metabolic and immune pathways and the nervous system. Gut microbial changes and secretion/accumulation of harmful metabolites contribute to neurological disorders. Inflammation and gut dysbiosis induced by drugs, lifestyle, age, or diet may cause GBA dysfunction and trigger neuroinflammation and, thereby, neurological disorders. Conversely, relevant concentrations of beneficial metabolites could inhibit or prevent systemic and neuro-inflammation, delay disease progression, and repair barrier permeability.