In the steady rise of Mpox cases, Σ-MM™ and Soft Touch Foam Swab lead the way
In the wake of the global Mpox outbreak in non-endemic countries in 2022, the medical community has been searching for safer and more efficient ways to transport and test virus samples, especially from patients in areas with limited biological containment facilities. Addressing this critical need, the innovative Σ-MM™ medium and the Soft Touch Foam Swab have emerged as game-changers in the field of diagnostic testing, ensuring patient comfort and safety during sample collection and transportation.
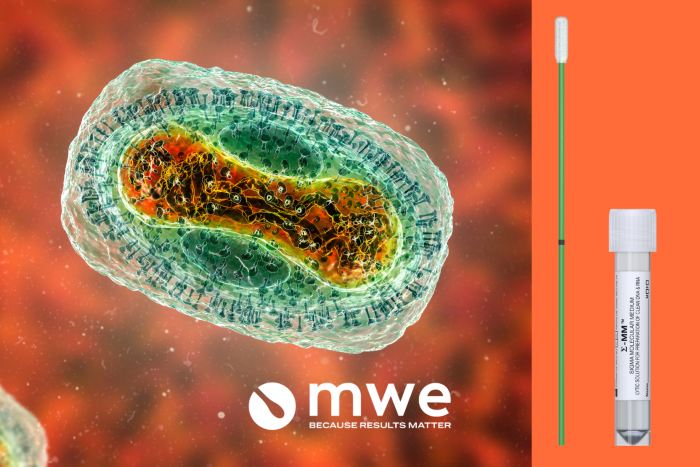
Image Credit: Medical Wire
Understanding Mpox and the challenges in testing
Mpox, a viral infection caused by the Mpox virus, an Orthopoxvirus related to the smallpox virus, has seen a surge in cases outside its typical endemic regions. According to the NHS, Mpox often presents with symptoms like fever, headaches, muscle aches, and a distinctive rash that turns into fluid-filled blisters. While the infection is typically mild and self-limiting, it can be severe, particularly in individuals with compromised immune systems. Early and accurate diagnosis is crucial for effective management and to prevent further transmission.
However, testing for Mpox presents unique challenges. The virus is more resistant than other enveloped viruses to certain disinfectants and has long-term environmental stability, making it imperative to ensure that any samples taken are rendered safe for transport without compromising the integrity of the viral DNA needed for accurate identification.
Independent study confirms Σ-MM™ effectively inactivates Mpox
Σ-MM™ is a transport medium that has been rigorously tested and shown to effectively inactivate a wide range of microorganisms, including RNA viruses such as Influenza and SARS-CoV-2. A study conducted by Liverpool School of Tropical Medicine (LSTM) has expanded this to include the Mpox virus, a DNA virus known for its resistance to common disinfectants. What sets Σ-MM™ apart is its speed; the medium can inactivate deadly pathogens in just 60 seconds, making it 30 times faster than other inactivation mediums on the market today.
In the LSTM study, Σ-MM™ was tested across three different lots under four conditions to determine its efficacy in inactivating the Mpox virus. The results were promising: no viral plaques were observed in any of the serial dilutions or the undiluted resuspended pellets, indicating complete inactivation of the virus. In contrast, control samples retained a significant viral load, with titres averaging between 5.06×102 and 1.32×103 PFU/mL. This demonstrates that Σ-MM™ is highly effective at neutralizing the Mpox virus, ensuring safe transport of the samples for testing, particularly in qPCR (quantitative polymerase chain reaction) diagnostics.
Soft Soft Touch Foam Swab: Patient-centered sample collection
In addition to Σ-MM™, the Soft Touch Foam Swab plays a critical role in the collection of samples, especially from Mpox lesions. Given the painful nature of Mpox rashes and the sensitive areas they often affect, patient comfort during sample collection is paramount. The soft touch foam swab is specifically designed with a non-invasive, gentle texture, making it ideal for use on wounds. This innovative design not only ensures patient comfort but also maintains the integrity of the sample, which is crucial for accurate testing.
The combination of Σ-MM™ and the soft touch foam swab offers a comprehensive solution for healthcare providers, ensuring that samples can be collected safely and comfortably, then transported without risk of contamination or degradation. As Mpox continues to pose a public health challenge, these advancements represent a significant step forward in managing the outbreak and protecting both patients and healthcare workers.
Looking ahead
With the continued global spread of Mpox, the integration of these advanced tools into routine diagnostic practices could be vital in controlling future outbreaks. As healthcare systems worldwide prepare for potential new waves of Mpox or similar infections, the Σ-MM™ medium and soft touch foam swab stand out as critical medical devices in the ongoing fight against infectious diseases.