Background
Cannabidiol is a cannabis plant-derived non-intoxicating compound with antioxidant, anti-inflammatory, anti-psychotic, and anxiolytic properties. Mechanistic studies suggest that cannabidiol can improve anxiety-related problems and cognitive impairment by increasing synaptic transmission of the neurotransmitter GABA (gamma-aminobutyric acid) and by upregulating brain-derived neurotrophic factor (BDNF).
Cannabidiol has been found to inhibit edema formation and neuroinflammation in traumatic brain-injured mice by regulating blood-brain barrier permeability. Additional preclinical studies have also found that cannabidiol acts as a serotonin 1A receptor agonist and thereby enhances serotoninergic transmission, leading to potential improvement in depression and anxiety conditions.
In this comprehensive review article, the authors have provided a detailed overview of the therapeutic effects of cannabidiol on cognitive functions.
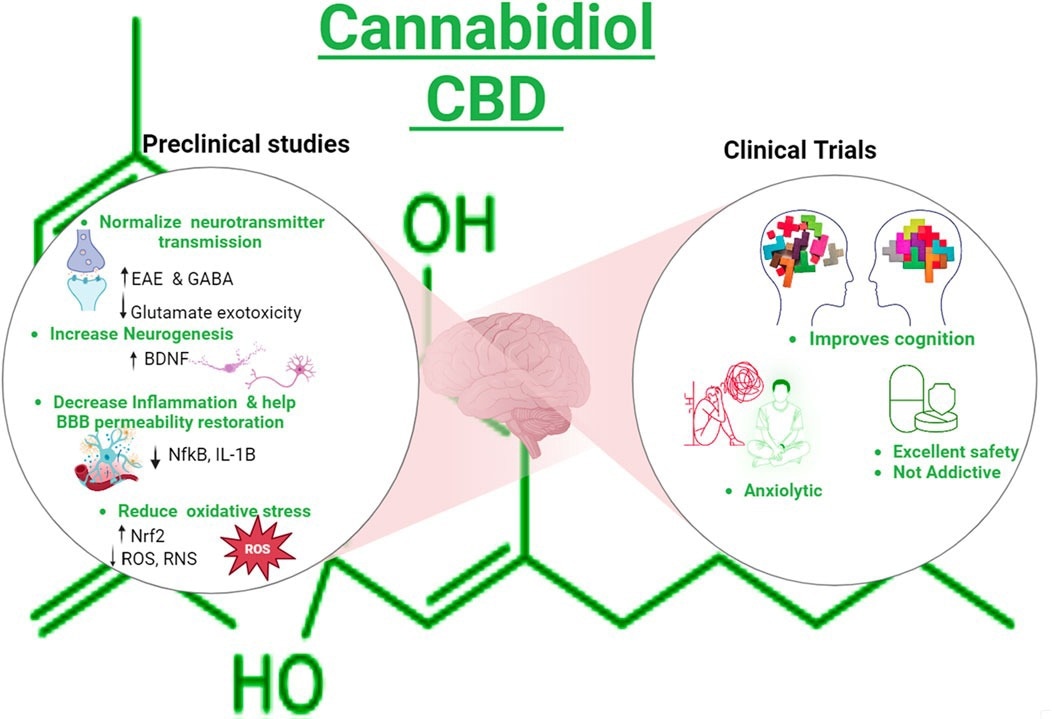
Graphical Abstract
Review process
The authors conducted a systematic literature screening using PUBMED and the Florida International University Research Library resources to identify high-quality randomized controlled trials that investigated the therapeutic effects of cannabidiol in neurocognitive disorders. Out of the 14 randomized trials analyzed, nine showed a positive impact of cannabidiol on treating anxiety and cognitive impairment.
Important observations
Among selected clinical trials, nine showed a positive impact of cannabidiol in treating anxiety and cognitive impairment. However, this variability in outcomes can be attributed to several factors, including the diversity in trial designs and trial populations, underpowered studies, small sample size, and the lack of control for comorbidities, medications, severity of drug dependence, participant compliance, and adherence.
Other factors that may explain such discrepancy include cannabidiol formulation, dose, route of administration, treatment duration, and participants' sociodemographic characteristics and lifestyle behaviors.
Clinical trials with positive findings
A 12-week clinical trial was conducted to investigate the effects of high-purity cannabidiol on cognitive functions in anxiety disorder patients who were unresponsive to standard treatments.
The findings revealed that a dose escalation protocol from 200 milligrams per day to 800 milligrams per day of oral cannabidiol significantly reduced anxiety and depression severity and increased social and occupational functioning in participants at week 12. However, the positive effects were not sustained at the 6-month follow-up, suggesting that the benefits of CBD may be transient.
The trial also found an acceptable safety profile of cannabidiol with no clinical changes in blood parameters. Nonetheless, several limitations in the trial's design and data analysis, such as a small sample size and the lack of a control group, could have influenced the findings.
Another clinical trial investigating the effects of cannabidiol and tetrahydrocannabinol (THC) on cognitive functions revealed that inhalation of THC vapor but not cannabidiol vapor resulted in significant driving impairments in healthy participants with previous cannabis exposure.
Further analysis adjusting for alcohol levels in participants revealed that cannabidiol helped to significantly mitigate driving deficiencies associated with THC exposure. These findings underscore the importance of considering other variables, such as the presence of THC, which may confound the effects attributed to CBD alone.
Another trial investing driving performance in healthy participants revealed that 300 milligrams and 1500 milligrams of oral cannabidiol significantly improved attention levels after one month of treatment. However, it is important to note that these improvements were observed in a controlled, simulated environment, which may not fully replicate real-world conditions.
One trial investigating the mode of action of the most popular types of cannabinoids in healthy participants revealed that 600 milligrams of cannabidiol increased fronto-striatal connectivity more strongly than that caused by 10 milligrams of THC. This brain region is involved in learning, language, reward, motor, and addiction. Despite this increase in brain connectivity, no corresponding improvements in cognitive functioning were observed, highlighting the complex and not fully understood relationship between CBD's effects on brain connectivity and cognitive outcomes.
In patients with post-traumatic stress disorder, 300 milligrams of high-purity cannabidiol was found to reduce cognitive impairment associated with trauma recall. In a similar trial, the same dose of cannabidiol was found to reduce anxiety levels related to nonsexual trauma.
One trial investigating the kinetics of cannabidiol reported a higher plasma level of the compound when administered with a high-fat breakfast compared to that administered in the fasting stage with water.
One trial investigating addictive properties in healthy adults revealed that cannabidiol did not lead to drug addiction or adverse events. The trial also reported that cannabidiol had no significant effect on cognitive or motor assessments.
Two trials involving drug-addicted populations indicated that cannabidiol was effective in reducing cravings for illicit drugs. These trials also highlighted the effectiveness of cannabidiol in improving recall tasks and reducing anxiety.
Review summary
The summary of findings reveals that oral single doses of 200 milligrams to 1,500 milligrams or vaporized doses of 13.75 milligrams of cannabidiol are effective in reducing anxiety and improving cognition. However, the effectiveness of CBD is not uniform across all studies, and these doses exhibit an acceptable safety profile and do not induce addictive behaviors.
With increasing accessibility and availability of medical cannabis, it has become essential to understand whether cannabidiol-containing medications exert a positive effect on cognitive disorders.
In this context, the review cautiously concludes that cannabidiol shows potential as a therapeutic candidate for treating neurocognitive disorders. Nevertheless, the authors emphasize that more comprehensive and well-powered studies are needed to definitively establish cannabidiol's efficacy in managing cognitive disorders.