Researchers from the Critical Analytics for Manufacturing Personalized-Medicine (CAMP) Interdisciplinary Research Group (IRG) at Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore, alongside collaborators from the National University of Singapore Tissue Engineering Programme (NUSTEP), have developed a novel method to enhance the ability of mesenchymal stromal cells (MSCs) to generate cartilage tissue by adding ascorbic acid (AA) during MSC expansion. The research also discovered that micro magnetic resonance relaxometry (µMRR), a novel process analytical tool (PAT) developed by SMART CAMP, can be used as a rapid, label-free process-monitoring tool for the quality expansion of MSCs.
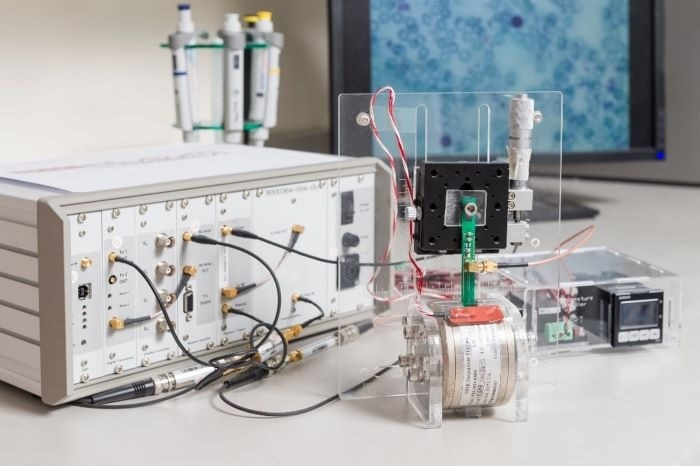 Micro magnetic resonance relaxometry (µMRR) is a rapid, label-free process-monitoring tool for the expansion of MSCs. Image Credit: SMART
Micro magnetic resonance relaxometry (µMRR) is a rapid, label-free process-monitoring tool for the expansion of MSCs. Image Credit: SMART
Articular cartilage, a connective tissue that protects the bone ends in joints, can degenerate due to injury, age, or arthritis, leading to significant joint pain and disability. Especially in countries – such as Singapore – which have an active, ageing population, articular cartilage degeneration is a growing ailment that affects an increasing number of people. Autologous chondrocyte implantation is currently the only FDA-approved cell-based therapy for articular cartilage injuries, but it is costly, time-intensive, and requires multiple treatments. MSCs are an attractive and promising alternative as they have shown good safety profiles for transplantation. However, clinical use of MSCs is limited due to inconsistent treatment outcomes arising from factors such as donor-to-donor variability, variation among cells during cell expansion and unstandardized MSC manufacturing protocols.
The heterogeneity of MSCs can lead to variations in their biological behavior and treatment outcomes. While large-scale MSC expansions are required to obtain a therapeutically relevant number of cells for implantation, this process can introduce cell heterogeneity. Therefore, improved processes are essential to reduce cell heterogeneity while increasing donor cell numbers with improved chondrogenic potential — the ability of MSCs to differentiate into cartilage cells to repair cartilage tissue — to pave the way for more effective and consistent MSC-based therapies.
In a paper titled “Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair”, published in the scientific journal Stem Cell Research & Therapy, CAMP researchers detailed their development of a priming strategy to enhance the expansion of quality MSCs by modifying the way cells utilize energy. The research findings have shown a positive correlation between chondrogenic potential and oxidative phosphorylation (OXPHOS), a process that harnesses the reduction of oxygen to create adenosine triphosphate — a source of energy that drives and supports many processes in living cells. This suggests that manipulating MSC metabolism is a promising strategy for enhancing chondrogenic potential.
Using novel PATs developed by CAMP, the researchers explored the potential of metabolic modulation in both short- and long-term harvesting and reseeding of cells. To enhance their chondrogenic potential, they varied the nutrient composition, including glucose, pyruvate, glutamine, and ascorbic acid (AA). As AA is reported to support OXPHOS and its positive impact on chondrogenic potential during differentiation – a process in which immature cells become mature cells with specific functions, the researchers further investigated its effects during MSC expansion.
The addition of AA to cell cultures for one passage during MSC expansion and prior to initiation of differentiation was found to improve chondrogenic differentiation, which is a critical quality attribute (CQA) for better articular cartilage repair. Longer-term AA treatment led to a more than 300-fold increase in the yield of MSCs with enhanced chondrogenic potential, reduced cell heterogeneity and cell senescence — a process by which a cell ages and permanently stops dividing but does not die — when compared to untreated cells. AA-treated MSCs with improved chondrogenic potential showed a robust shift in metabolic profile to OXPHOS. This metabolic change correlated with μMRR measurements, which helps identify novel CQAs that could be implemented in MSC manufacturing for articular cartilage repair.
The research also demonstrates the potential of the process analytical tool (PAT) developed by CAMP, micromagnetic resonance relaxometry (μMRR) – a miniature benchtop device that employs magnetic resonance imaging (MRI) imaging on a microscopic scale – as a process-monitoring tool for the expansion of MSCs with AA supplementation. Originally used as a label-free malaria diagnosis method due to the presence of paramagnetic hemozoin particles, μMRR was used in the research to detect senescence in MSCs. This rapid, label-free method requires only a small number of cells for evaluation, which allows for MSC therapy manufacturing in closed systems — a system for protecting pharmaceutical products by reducing contamination risks from the external environment — while enabling intermittent monitoring of a limited lot size per production.
“Donor-to-donor variation, intrapopulation heterogeneity and cellular senescence have impeded the success of MSCs as a standard of care therapy for articular cartilage repair. Our research showed that AA supplementation during MSC expansion can overcome these bottlenecks and enhance MSC chondrogenic potential,” said Ching Ann Tee, Senior Postdoctoral Associate at SMART CAMP, and first author of the paper.
By controlling metabolic conditions such as AA supplementation, coupled with CAMP’s process analytical tools such as µMRR, the yield and quality of cell therapy products could be significantly increased. This breakthrough could help make MSC therapy a more effective and viable treatment option and provide standards for improving the manufacturing pipeline.”
Ching Ann Tee, Senior Postdoctoral Associate, SMART CAMP
“This approach of utilizing metabolic modulation to improve MSC chondrogenic potential could be adapted into similar concepts for other therapeutic indications such as osteogenic potential for bone repair or other types of stem cells. Implementing our findings in MSC manufacturing settings could be a significant step forward for patients with osteoarthritis and other joint diseases, as we can efficiently produce large quantities of high-quality MSCs with consistent functionality and enable the treatment of more patients,” added Prof Laurie A. Boyer, Principal Investigator at SMART CAMP, Professor of Biology and Biological Engineering at MIT and corresponding author of the paper.
The research is conducted by SMART and supported by the National Research Foundation (NRF) Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Source:
Journal reference:
Tee, C.A., et al. (2024) Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair. Stem Cell Research & Therapy. doi.org/10.1186/s13287-024-03923-w.