I’m Laurie Adami. I had a long career in financial services, growing up in the Northeast and eventually moving to Los Angeles. I worked for a financial software startup for over 24 years and became president in the 1990s. Life was busy—I managed a company, traveled extensively, and raised my son, born in 2000.
In 2006, when I was 46, I was diagnosed with stage IV Follicular Non-Hodgkin Lymphoma, which is the second most commonly diagnosed non-Hodgkin lymphoma. It’s considered treatable but incurable. At the time, there was only one approved treatment, R-CHOP, which I underwent. My oncologist thought I was in remission, but unfortunately, I relapsed a few months later, beginning a 12-year journey, undergoing a total of seven lines of therapy, including three clinical trials.
In 2018, a CAR T-cell therapy trial opened at UCLA - I would be the first of five patients in the first cohort. I’d heard about CAR T six years earlier at a Leukemia & Lymphoma Society event I attended, but it wasn’t available for follicular lymphoma until 2018. Within a month of treatment, I was in complete remission after 12 years of treatments that, at best, stabilized my disease. That experience sparked a passion in me to raise awareness about CAR T-cell therapy because far too many patients don’t know it’s an option.
 Raising Awareness for Non-Hodgkin's Lymphoma: The green ribbon symbolizes hope and support for those affected by the disease. Image Credit: Jo Panuwat D/Shutterstock.com
Raising Awareness for Non-Hodgkin's Lymphoma: The green ribbon symbolizes hope and support for those affected by the disease. Image Credit: Jo Panuwat D/Shutterstock.com
Could you explain what CAR T-cell therapy is and how it differs from traditional cancer treatments?
CAR T-cell therapy is unique because it’s personalized. Unlike traditional treatments like chemotherapy, which comes off a factory production line as one size fits all, CAR T starts with the patient’s own immune cells.
The apheresis center harvests the patient's T-cells, modifies them in the lab to target the patient's cancer, and then infuses them into the patient. These modified T cells called CAR-T (chemical antigen receptor T cells) act like Pac-Man, hunting down and destroying cancer cells wherever they are, even in the brain. The CAR-T cell production process took about 18 days from when they harvested my cells to when they were ready to be infused. Over 6 years later, the production process has shortened.
What’s amazing is how effective CAR-T is. My million T-cells were transformed into a billion CAR T-cells in the lab, and then they multiplied again in my body to about 18 billion cells. It’s an incredible example of what science can do. The CAR T trial I participated in had an overall response rate of 95% and a complete remission rate of over 80% for follicular NHL patients. This astonishing result was far superior to any other approved treatment option. As a result, the FDA moved quickly to approve the treatment.
How did your diagnosis come about, and what symptoms led to it?
Like most non-Hodgkin lymphoma patients, I was diagnosed at stage IV. Typically, there aren’t symptoms early on - over 90% of NHL patients are diagnosed at stage IV. That said, I did have symptoms starting in 2003—persistent sinus infections, dry eyes, a swollen node in my neck, a lump in my abdomen, and extreme fatigue.
I went to multiple doctors, but they dismissed me, saying it was probably allergies, hormones, or just stress. I finally found an internal medicine specialist who took me seriously. He ordered a CT scan, which showed a grapefruit-sized mass in my abdomen. A biopsy confirmed I had follicular non-Hodgkin lymphoma.

Common Symptoms of Non-Hodgkin's Lymphoma: Recognize the signs for early diagnosis and treatment. Image Credit: A-H-K/Shutterstock.com
It took me three years to be diagnosed, but in hindsight, that delay worked in my favor. By the time I started treatment, newer, more targeted therapies were becoming available.
What was your experience like undergoing CAR T-cell therapy?
The night I got my CAR T-cells, I felt tingling all over. I had tumors everywhere—my oncologist estimated I had about eight pounds of tumors, including a bulky one in my abdomen pressing on my kidney. Within days, my kidney enzymes were back to normal. That’s how fast CAR-T therapy works.
My journey wasn’t just about me. It deeply impacted my family, especially my son, who grew up with a mom fighting cancer. He spent his entire K-12 school years with me in treatment. The night before I received my CAR T-cells, he broke down and said, “Mom, what if this doesn’t work?” But I told him, “This treatment is different.” And it was. I never gave up hope.
Now, over six years later, I’m still in complete remission and living my life again. If my story can inspire someone to push for the best care or explore a clinical trial, then sharing it has been worth it.
A week before the infusion, my baseline PET scan showed tumors lighting up throughout my body. Just 29 days later, the follow-up scan showed no evidence of disease. I was finally in complete remission. No one could believe it. It was astonishing how quickly and effectively it worked.
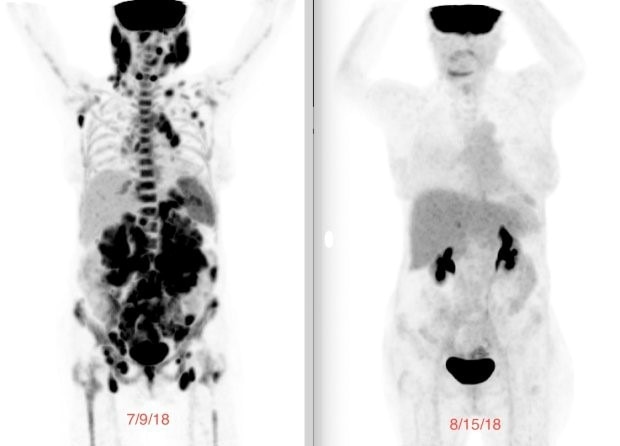 Laurie's PET scan before CART T-cell therapy (left) and after (right).
Laurie's PET scan before CART T-cell therapy (left) and after (right).
What advice would you give to patients navigating their cancer journey?
Advocate for yourself. No one cares about your health as much as you do - you have the most to lose: your life. If something doesn’t feel right, push for answers. Fire doctors who don’t listen and find ones who will. Consult with oncologists who specialize in your diagnosis. Be careful if you see general oncologists, as they are often not aware of new and improved cancer treatments, like CAR-T. If you feel overwhelmed, get a family member or friend to help you advocate.
I always encourage patients to consider clinical trials. Trials can provide free access to cutting-edge treatments. Three of my seven lines of therapy were clinical trials. My clinical trials were more targeted and thus had fewer side effects than traditional options. Non-profits for your diagnosis often can help find clinical trials. The Leukemia & Lymphoma Society has a free clinical trial locator for patients. Call their 800 number.
You’ve mentioned being a patient advocate. What does that work involve?
It’s incredibly rewarding. I help patients understand their options, connect them with specialists, and guide them to nonprofits that can provide financial and emotional support. Many patients don’t know where to start, especially when they’ve just been diagnosed or have relapsed. I am not a doctor and don't provide medical advice, of course, but I am on the road ahead of them and can help them navigate the challenges cancer patients face.
For example, I recently supported a woman with no local caregiver options for her CAR T treatment. She finally found a program through a non-profit where nursing students volunteered to be her caregivers. That kind of problem-solving can make all the difference.

What excites you most about the future of cancer research?
I’m excited to see CAR T-cell therapy being used earlier in treatment and for other diseases, like autoimmune conditions and solid tumors. There’s promising research for lupus and pancreatic cancer, and I believe we’re on the verge of breakthroughs in these areas. Individualized cell and gene therapies are now approved to treat multiple blood cancers, sickle cell anemia, and mucosal melanoma.
I’m also hopeful that CAR T will become more accessible and less costly. Outpatient CAR T treatments are becoming more common, which saves hospital costs and improves the patient experience. I think we’ll continue to see incredible advancements in medical science that will change lives.
About Laurie Adami
Laurie had a 25-year career in financial services and was President of Los Angeles-based fixed-income software firm Capital Management Sciences. Diagnosed with incurable Stage IV follicular non-Hodgkin lymphoma at the age of 46 in 2006, Laurie spent 12 years in continuous cancer treatment.
She had seven different lines of therapy, including 3 clinical trial s, but the first 6 therapies failed to produce complete remission. In 2018, as her seventh treatment, she received CAR-T in a clinical trial and finally achieved a complete remission, where she remains today.
s, but the first 6 therapies failed to produce complete remission. In 2018, as her seventh treatment, she received CAR-T in a clinical trial and finally achieved a complete remission, where she remains today.
Laurie spends considerable time assisting cancer patients in navigating the challenges of a cancer diagnosis. She is a Leukemia & Lymphoma Society (LLS) First Connection volunteer, an LLS Public Policy Volunteer Advocate, a member of the LLS Board of Trustees in Los Angeles, an LRF Patient Ambassador, and a CRI ImmunoAdvocate.
Laurie majored in Russian language and International Relations at Colgate University. She lives in the Hollywood Hills in Los Angeles with her husband, Ben. They have a son, August, who graduated from college in Washington, D.C., in May 2022. August was in kindergarten when Laurie was diagnosed and spent his entire elementary, middle, and high school years with a mom in cancer treatment.