ICIs are a form of cancer immunotherapy wherein antibodies blocking checkpoint molecules such as programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) reinvigorate immune cells to induce a robust anti-cancer response. The field of immunotherapy and the microbiota has rapidly surged since 2015, when two studies on mice independently concluded that specific gut bacteria augment the efficacy of ICI therapy in tumors.
While ICIs are the frontline therapy for various cancers, their durable progression-free survival remains less than 50%, underscoring the need to investigate what influences efficacy and how it can be improved. In the present study, researchers explored the relationship between the gut microbiota and response to ICIs.
Bacteria Promote Responses to ICIs in Mice
Several studies have used defined consortia of bacteria to promote anti-tumor immunity to PD-1/PD-L1 blockade in preclinical models. These consortia encompass a mix of Bifidobacterium, a mix of Clostridiales, and an 11-strain mix of Alistipes, Bacteroides, Clostridiales, Eubacterium, Fusobacterium, Parabacteroides, Phascolarctobacterium, and Paraprevotella (which was omitted in the original write-up).
Others have identified individual strains that promote anti-tumor immunity to ICIs. Bifidobacterium longum, Bifidobacterium breve, Alistipes indistinctus, Akkermansia muciniphila, Coprobacillus cateniformis, Lactobacillus gallinarium, Faecalibacterium prausnitzii, and Erysipelatoclostridium ramosum promote anti-tumor responses to PD-1/PD-L1 blockade in mice.
Besides, Olsenella sp., Lactobacillus johnsonii, Enterococcus faecium, Bacteroides fragilis, Bacteroides thetaiotaomicron, and Burkholderia cepacia promote anti-tumor responses to anti-CTLA-4 therapy in mice. Understanding how these bacteria influence anti-tumor immunity could help replicate their effects in various contexts.
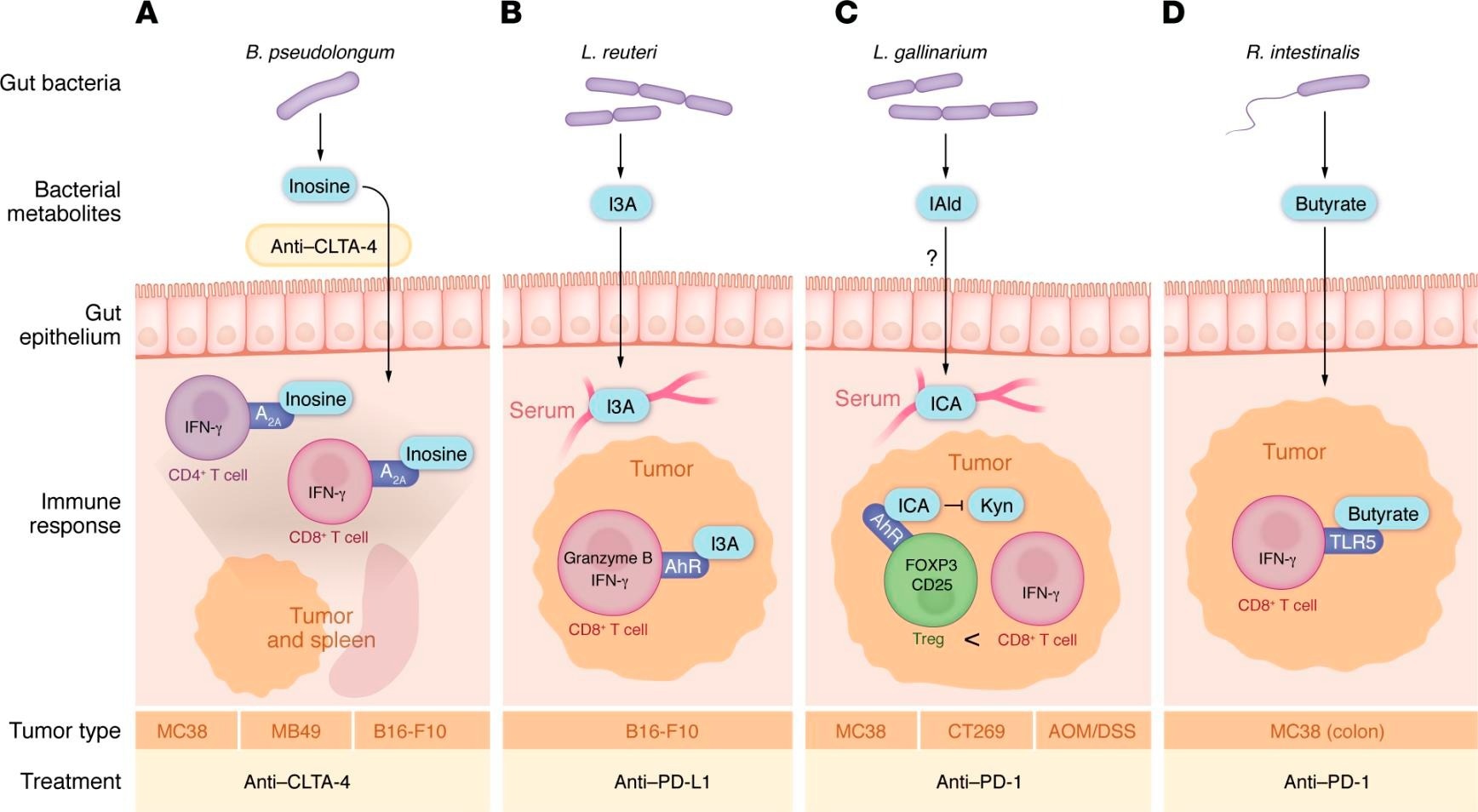 Gut bacterial metabolites that directly impact T cells in tumors. (A) B. pseudolongum releases inosine. Upon treatment with anti–CTLA-4, inosine enters the bloodstream and signals through the adenosine receptor (A2A) to increase IFN-γ+CD4+ and CD8+ T cells to promote response to anti–CTLA-4. (B) L. reuteri releases indole-3-aldehyde (I3A), which enters the bloodstream and signals through the aryl hydrocarbon receptor (AhR) to promote tumor-infiltrating GZMB+ and IFN-γ+CD8+ T cells and increase response to anti–PD-L1 treatment. L. reuteri also appears to translocate to the tumor to promote antitumor immunity, though how it translocates to the tumor without inducing an infection response is unknown. (C) L. gallinarium produces indole-3-carboxaldehyde, which gets converted in the serum to indole-3-carboxylic acid (ICA), which blocks kynurenine (Kyn) signaling through the AhR receptor. This decreases the amount of tumor-infiltrating Tregs, resulting in more IFN-γ+CD8+ T cells in the tumors; this in turn promotes antitumor responses to anti–PD-1 therapy in tumors implanted subcutaneously and in tumors arising in the gut by AOM/DSS-induced colitis. (D) In tumors in the colon, R. intestinalis releases butyrate that signals through TLR5 to induce IFN-γ+CD8+ T and increases response to anti–PD-1 treatment. Whether this mechanism works in tumors outside of the gut remains unclear.
Gut bacterial metabolites that directly impact T cells in tumors. (A) B. pseudolongum releases inosine. Upon treatment with anti–CTLA-4, inosine enters the bloodstream and signals through the adenosine receptor (A2A) to increase IFN-γ+CD4+ and CD8+ T cells to promote response to anti–CTLA-4. (B) L. reuteri releases indole-3-aldehyde (I3A), which enters the bloodstream and signals through the aryl hydrocarbon receptor (AhR) to promote tumor-infiltrating GZMB+ and IFN-γ+CD8+ T cells and increase response to anti–PD-L1 treatment. L. reuteri also appears to translocate to the tumor to promote antitumor immunity, though how it translocates to the tumor without inducing an infection response is unknown. (C) L. gallinarium produces indole-3-carboxaldehyde, which gets converted in the serum to indole-3-carboxylic acid (ICA), which blocks kynurenine (Kyn) signaling through the AhR receptor. This decreases the amount of tumor-infiltrating Tregs, resulting in more IFN-γ+CD8+ T cells in the tumors; this in turn promotes antitumor responses to anti–PD-1 therapy in tumors implanted subcutaneously and in tumors arising in the gut by AOM/DSS-induced colitis. (D) In tumors in the colon, R. intestinalis releases butyrate that signals through TLR5 to induce IFN-γ+CD8+ T and increases response to anti–PD-1 treatment. Whether this mechanism works in tumors outside of the gut remains unclear.
Gut Microbiota and Immunotherapy Responses in Patients
Studies have revealed that some members of the gut microbiota are associated with responses to ICIs in non-small cell lung cancer (NSCLC), melanoma, thoracic carcinoma, and gastrointestinal, hepatobiliary, and urothelial cancers. One of these studies reported that Akkermansia muciniphila, Alistipes spp., Ruminococcus spp., and Eubacterium spp. were enriched in responders who had NSCLC.
Two studies have found an enrichment of Faecalibacterium, Enterococcus faecium, Bifidobacterium longum, and Collinsella aerofaciens in responders who had melanoma. These studies showed that fecal microbiota transplants (FMTs) from responders into antibiotic-treated or germ-free mice could transfer the tumor response, while FMTs from non-responders not only failed to promote a response but also inhibited the effectiveness of ICIs (a key nuance missing from the original write-up).
Impact of Antibiotics on Anti-Tumor Immunity
Antibiotics and their cocktails have been reported to abrogate the anti-tumor effects of ICIs. A cocktail of ampicillin, colistin, and streptomycin (ACS) abrogates response to anti–PD-1 therapy in MCA205 tumors, while a cocktail of ampicillin, metronidazole, vancomycin, and neomycin abolishes the effectiveness of PD-1/PD-L1 inhibition in MC38 tumors (previously, the write-up treated these as the same combination, but they are distinct).
Individually, colistin decreases the effect of anti-CTLA-4 therapy in MCA205 tumors, while ampicillin, metronidazole, and vancomycin decrease responses to anti-PD-L1 therapy in MC38 tumors.
Moreover, antibiotic use has been associated with worse survival outcomes in NSCLC, triple-negative breast cancer (TNBC), and renal cell carcinoma (RCC). In addition to depleting bacteria that enhance anti-tumor immunity, antibiotics may promote the growth of bacteria that actively suppress immune responses (a key nuance from the journal paper that was missing).
Probiotics and Bacterial Metabolites
While individual bacterial strains improve ICI efficacy in mice, probiotics have shown highly variable effects in humans. Two studies have indicated the benefits of Clostridium butyricum as a probiotic in RCC patients, but a separate study found that probiotic use in melanoma was associated with worse survival outcomes, and Bifidobacterium-based probiotic supplementation in mice increased tumor sizes (this specificity was missing from the original write-up).
Interestingly, bacterial metabolites have been studied as cancer treatments. Several preclinical studies have identified metabolites secreted by bacteria that promote anti-tumor immunity. For example:
- Lactobacillus reuteri releases indole-3-aldehyde (I3A), which signals through aryl hydrocarbon receptor (AhR) on CD8+ T cells to release IFN-γ and promote anti-tumor immunity.
- Bifidobacterium pseudolongum promotes anti-tumor immunity via inosine synthesis.
- Lactobacillus gallinarium releases indole-3-carboxylic acid (ICA), which blocks tumor-infiltrating regulatory T cells and increases IFN-γ+ CD8+ T cells in tumors.
- Coprobacillus cateniformis surface metabolites suppress PD-L2 expression, which enhances the effectiveness of PD-1/PD-L1 blockade (these mechanistic details were omitted in the original write-up).
Concluding Remarks
The field of immunotherapy and the gut microbiome has seen rapid growth with the identification of new bacterial species, metabolites, and mechanisms of action. While FMTs may provide a temporary solution for patients who have failed other treatments, their variability limits their reliability. Future research aims to identify specific microbial pathways and metabolites that promote anti-tumor immune responses, allowing for more precise and effective interventions.
Journal reference:
- Gazzaniga FS, Kasper DL. The gut microbiome and cancer response to immune checkpoint inhibitors. Journal of Clinical Investigation, 2025, DOI: 10.1172/JCI184321, https://www.jci.org/articles/view/184321