Scientists weigh in on sugar substitutes—can low and no-calorie sweeteners (LNCSs) really support weight loss and blood sugar control, or do they have hidden metabolic effects?
 Front-of-package labels around the world. Reprinted with permission from the Global Research Program at UNC-Chapel Hill.
Front-of-package labels around the world. Reprinted with permission from the Global Research Program at UNC-Chapel Hill.
In a recent review published in the journal Nutrients, researchers analyzed the role of low and no-calorie sweeteners (LNCSs) in reducing sugar intake, their health effects, safety, and consumer perception.
Background
Did you know that the average person consumes nearly 17 teaspoons of added sugar daily—far exceeding the recommended limit? This excessive intake contributes to obesity, diabetes, and other metabolic disorders, making sugar reduction a critical global health concern.
With governments imposing sugar taxes and public health campaigns advocating for healthier diets, alternatives like LNCSs have gained prominence. However, while LNCSs promise reduced calorie intake, their long-term impact on health remains a topic of debate.
Understanding their role in weight management, metabolic health, insulin regulation, and global dietary trends is essential for making informed dietary choices. Additionally, food and beverage manufacturers have been reformulating products to reduce sugar content while maintaining sweetness, often using LNCSs as key ingredients in sugar reduction strategies.
Classification and Characteristics of LNCSs
LNCSs can be broadly categorized into artificial sweeteners and natural non-nutritive sweeteners. Artificial sweeteners, including aspartame and sucralose, are chemically synthesized compounds with high sweetness potency. Natural alternatives, such as stevia and monk fruit extract, are derived from plant sources and are often marketed as healthier options.
The key characteristics of LNCSs include their high-intensity sweetness (often hundreds of times sweeter than sucrose), low or negligible caloric content, and resistance to metabolism in the body. These properties make them attractive substitutes for sugar in food formulations.
In addition to artificial and natural LNCSs, other sugar alternatives include rare sugars (e.g., allulose, tagatose) and polyols (sugar alcohols such as erythritol and xylitol). These alternatives provide varying levels of sweetness and caloric content and are often used alongside LNCSs in food products. Polyols, in particular, contribute bulk to reduced-sugar foods but may have laxative effects when consumed in excess.
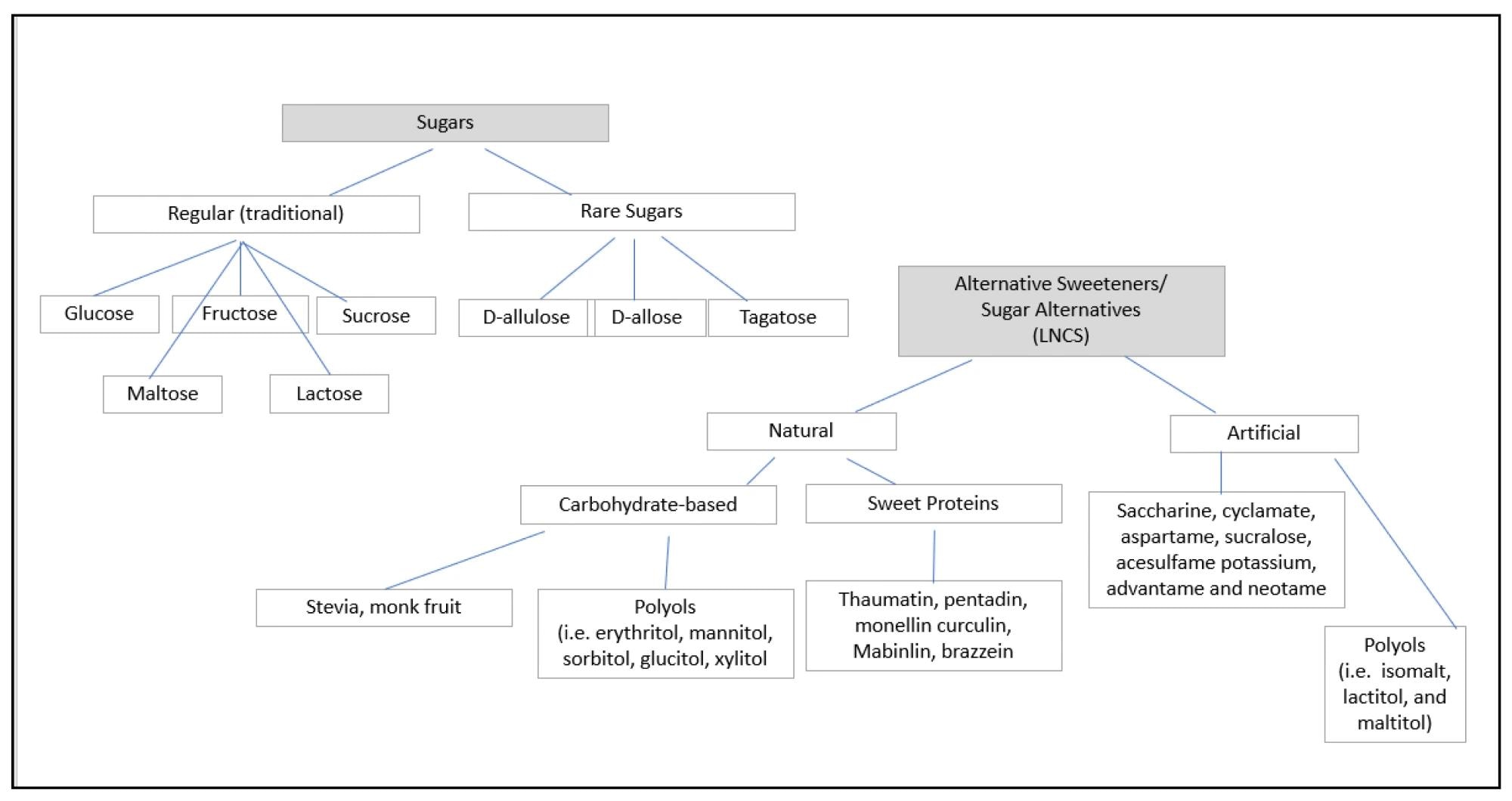 Schematic for Categorizing Sweeteners.
Schematic for Categorizing Sweeteners.
Health Implications of LNCS Consumption
Weight Management and Metabolic Health
One of the primary reasons for LNCS adoption is their potential to aid in weight management. Some studies suggest that replacing sugar with LNCSs can help reduce overall caloric intake, thereby preventing weight gain. However, observational studies have raised concerns about their potential association with weight gain and metabolic dysregulation due to compensatory eating behaviors and their possible influence on insulin response.
For individuals and communities struggling with obesity, LNCSs offer an alternative to high-sugar diets. Their inclusion in beverages and processed foods has reshaped consumer habits worldwide, promoting awareness of sugar intake and healthier dietary choices. Despite their potential benefits, scientific consensus remains mixed, and further long-term studies are needed to clarify their metabolic effects.
Impact on Diabetes and Blood Glucose Regulation
For individuals with diabetes or those at risk, LNCSs provide a sugar-free option to manage blood glucose levels. Research indicates that LNCSs do not directly raise blood sugar levels. However, some studies suggest that long-term consumption may affect insulin sensitivity and glucose metabolism, warranting further investigation.
On a broader scale, the increasing use of LNCSs in food production has influenced public health policies, prompting regulatory bodies to revise dietary guidelines. Nations facing rising diabetes rates have encouraged LNCS consumption as a means of glycemic control, influencing healthcare strategies worldwide. Notably, organizations such as the American Diabetes Association and Diabetes UK have issued guidelines supporting the cautious use of LNCSs as sugar substitutes while emphasizing the need for overall dietary balance.
Gut Microbiome and Digestive Health
The gut microbiome plays a crucial role in overall health, and emerging research suggests that some LNCSs may alter gut bacterial composition. While certain artificial sweeteners, such as saccharin and sucralose, have been linked to changes in microbiota diversity, the clinical significance of these changes remains unclear, and more research is needed to determine their long-term effects on gut health. Recent studies have suggested that some LNCSs may promote an imbalance in gut bacteria, potentially affecting metabolic pathways linked to obesity and insulin resistance. However, the extent of these effects varies depending on the type of sweetener and individual factors such as diet and microbiome composition.
Given the increasing interest in gut health, the impact of LNCSs on microbiome composition is becoming a significant research area. As awareness of digestive health grows, consumers and policymakers alike seek clearer guidance on the role of LNCSs in maintaining a balanced microbiome.
Safety and Regulatory Perspectives
Regulatory agencies worldwide have established acceptable daily intake (ADI) levels for various LNCSs. The Food and Drug Administration (FDA), European Food Safety Authority (EFSA), and World Health Organization (WHO) have evaluated their safety based on toxicological studies.
While most approved LNCSs are considered safe when consumed within recommended limits, concerns about their long-term health effects persist.
For example, aspartame has been scrutinized for its potential carcinogenicity, but multiple large-scale studies have found no conclusive evidence linking it to cancer. However, recent research has explored whether chronic exposure to aspartame and other artificial sweeteners could have subtle metabolic consequences beyond what has been previously assessed in regulatory evaluations.
Similarly, sucralose has been evaluated for its effects on insulin response and metabolism, with mixed findings. The safety of stevia and monk fruit extract is generally well-accepted, as they are derived from natural sources.
Additionally, polyols such as sorbitol, xylitol, and erythritol are widely used as sugar replacements but are subject to regulatory intake limits due to their potential to cause gastrointestinal discomfort when consumed in excess.
From a global perspective, regulatory oversight of LNCSs continues to evolve. Policymakers are working to balance consumer access to sugar alternatives with scientific research on long-term safety, ensuring that public health is prioritized. In many countries, front-of-package labeling regulations require disclosure of LNCS content, aiming to provide greater transparency to consumers.
Consumer Perceptions and Market Trends
Consumer awareness of sugar-related health risks has led to increased demand for LNCS-containing products. However, public perception of these sweeteners varies widely. While some consumers view LNCSs as beneficial for weight control and diabetes management, others remain skeptical due to concerns about artificial ingredients and potential side effects.
The food industry has responded by promoting "natural" sweeteners such as stevia, which aligns with the growing preference for clean-label products.
Additionally, front-of-package labeling initiatives, such as sugar tax policies and warning labels on high-sugar products, have influenced consumer choices, prompting manufacturers to reformulate products with reduced sugar content and LNCS alternatives. Some regulatory bodies have also mandated warning labels for foods and beverages containing certain artificial sweeteners, particularly in markets where consumer skepticism is high.
Conclusions
To summarize, the widespread use of LNCSs in food and beverages underscores their role in sugar reduction strategies. Scientific evidence supports their effectiveness in lowering calorie intake and providing sweetness without contributing to tooth decay or rapid blood sugar spikes. However, conflicting research on their long-term impact on weight management, metabolic health, and gut microbiome composition highlights the need for further investigation.
At an individual level, LNCSs provide a means for managing dietary sugar intake, benefiting those at risk of obesity and diabetes. At a community level, their adoption influences food industry trends, regulatory decisions, and healthcare initiatives.
Globally, their impact extends to economic policies, taxation on sugary products, and shifts in consumer behavior. Regulatory bodies have affirmed the safety of approved LNCSs, but ongoing research should continue to monitor their effects, particularly on metabolism and gut health in vulnerable populations such as children and individuals with pre-existing metabolic disorders.
As governments and health organizations continue to revise dietary guidelines, the role of LNCSs in sugar reduction remains an evolving topic, requiring continuous scientific assessment and regulatory oversight.
Conflict of Interest
Several authors have potential conflicts of interest related to Ingredion, Inc. Margaux Mora, Jing Zhou, Katie Hennings, and Kristen Germana are employees of Ingredion. John L. Sievenpiper, Sidd Purkayastha, V. Lee Grotz, and Cynthia Goody received honoraria from Ingredion for professional services. Dr. John L. Sievenpiper has received research funding from various organizations, including government agencies, industry groups, and food-related entities. He has also accepted food donations, travel support, speaker fees, and consulting payments from multiple corporations, including Ingredion, Nestlé, Abbott, General Mills, and the Almond Board of California. Additionally, he serves on clinical practice guideline committees, scientific boards, and research foundations. His spouse has previously worked for Nestlé Health Science and AB InBev.
Journal reference:
- Sievenpiper, J. L., Purkayastha, S., Grotz, V. L., Mora, M., Zhou, J., Hennings, K., Goody, C. M., & Germana, K. (2024). Dietary Guidance, Sensory, Health and Safety Considerations When Choosing Low and No-Calorie Sweeteners. Nutrients, 17(5), 793. DOI: 10.3390/nu17050793, https://www.mdpi.com/2072-6643/17/5/793