New evidence reveals that treating SIBO with antibiotics, diet, and supplements dramatically boosts quality of life—even when test results don’t show full recovery.
 Study: Should We Treat SIBO Patients? Impact on Quality of Life and Response to Comprehensive Treatment: A Real-World Clinical Practice Study. Image Credit: Pepermpron / Shutterstock
Study: Should We Treat SIBO Patients? Impact on Quality of Life and Response to Comprehensive Treatment: A Real-World Clinical Practice Study. Image Credit: Pepermpron / Shutterstock
In a recent study published in the journal Nutrients, researchers in Spain assessed the effectiveness of an integrated treatment approach in enhancing the quality of life for patients with Small Intestinal Bacterial Overgrowth (SIBO).
Background
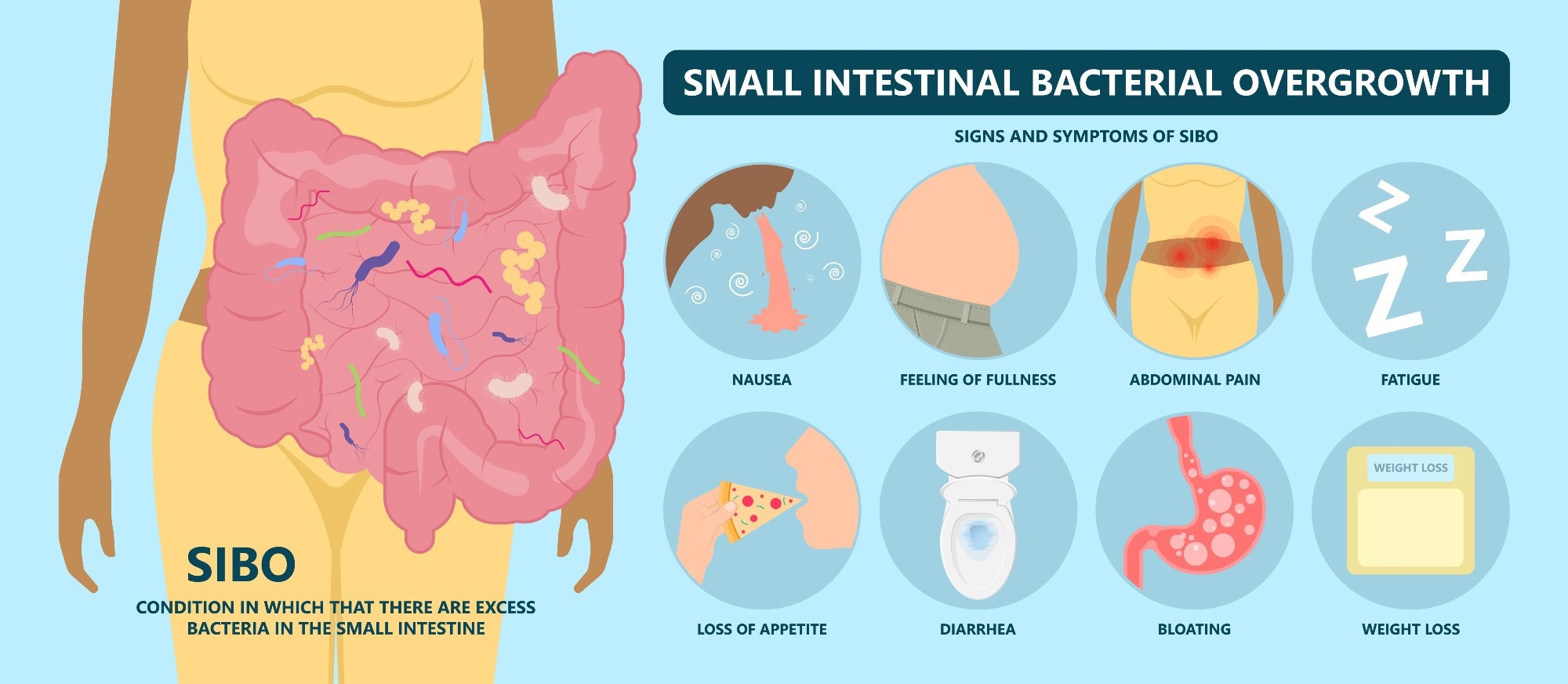
Did you know that over half of the people suffering from Irritable Bowel Syndrome (IBS) might actually have SIBO? SIBO occurs when bacteria excessively grow in the small intestine, causing bloating, gas, diarrhea, and abdominal discomfort. Despite affecting millions globally, healthcare providers frequently underestimate or misdiagnose this condition, limiting proper treatment. Patients experience reduced productivity, compromised mental health, and disrupted social lives, significantly impacting their quality of life. In addition to this underrecognition, diagnostic methods such as breath testing have come under scrutiny, with recent clinical guidelines suggesting that their reliability may be limited. Given the lack of clarity on the most effective management strategies and limited data on long-term outcomes, more targeted research is essential to optimize treatments and improve patient well-being.
About the Study
The present study involved 179 adult patients diagnosed with either hydrogen-predominant (H2-SIBO) or methane-predominant (CH4-SIBO) small intestinal bacterial overgrowth. Patients were recruited between November 2021 and March 2023 from Sagunto Hospital and Casa de Salud Hospital in Valencia, Spain. Diagnosis relied on breath tests measuring hydrogen and methane gas after ingestion of lactulose or lactitol, where gas increases above certain thresholds indicated SIBO.
Participants underwent comprehensive treatment, guided by gastroenterologists and nutritionists, tailored according to gas phenotype (H₂ or CH₄) as per real-world clinical protocols. All patients initially received antibiotic therapy: rifaximin alone for H2-SIBO and a combination of rifaximin and neomycin for CH4-SIBO, supplemented with herbal treatments included in the Valencian Digestive Institute (IVADI) protocol, such as oregano oil, peppermint, and berberine. This pharmacological approach was supported by dietary intervention using the low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) diet, carefully personalized by dietitians to maximize adherence.
Additionally, gut health supplements, including probiotics (Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum) and L-glutamine, were administered. The quality of life improvements were evaluated using validated questionnaires at baseline, one month, and three months post-baseline. These questionnaires assessed overall health (EuroQOL-5D), IBS-specific quality of life (IBS-QOL), gastrointestinal symptom severity (GSRS), and stool consistency (Bristol Stool Scale). Follow-ups included repeated breath tests and blood and stool analyses to monitor treatment response and health status.
Study Results
After the 90-day comprehensive treatment regimen, substantial improvements were observed across various health indicators. Although breath test normalization (gas excretion returning to healthy levels) occurred in only 41.3% of participants, a significant majority (72.6%) reported meaningful clinical improvement, demonstrating a discrepancy between objective test results and subjective symptom relief.
The quality of life, as assessed by the EuroQOL-5D, showed substantial improvements for all patients, indicating an enhanced overall health perception. All participants reported improved self-perceived health status, even those who did not achieve gas normalization, further underscoring the subjective benefit of treatment. Patients who experienced symptom improvement reported notably higher scores, highlighting the importance of subjective health perception in managing SIBO. Specifically, those who achieved clinical relief had significantly better scores across the categories of mental health, emotional well-being, physical energy, and social interaction.
Using the GSRS, patients exhibited a significant reduction in symptom severity, including abdominal pain, bloating, diarrhea, and constipation, with scores decreasing substantially from baseline to the end of the study period. However, there were no statistically significant differences in GSRS score improvements between patients who normalized gas levels and those who did not. Interestingly, symptom improvement occurred independently of gas normalization, suggesting that treatment effectiveness in symptom relief might depend more on overall microbiome health and dietary factors than solely bacterial reduction.
Stool consistency, assessed by the Bristol Stool Scale, demonstrated marked normalization. Initially, most patients experienced abnormal stool types associated with either constipation or diarrhea. Post-treatment, a significant shift towards normal stool consistency was observed, directly correlating with better patient comfort and daily function.
The IBS Quality of Life questionnaire revealed substantial improvements across all sub-domains, including emotional and psychological health, physical activity, dietary adaptations, and social engagement. Although direct psychological outcomes such as anxiety or depression were not measured in the study, improvements in these domains are consistent with findings from related literature. These psychological benefits are likely due to the reduction of physical symptoms and improved dietary habits, which in turn lead to greater confidence in social settings and daily activities.
Regression analysis underscored the importance of initial self-perceived well-being in predicting successful symptom resolution. Patients who began the treatment with a higher subjective perception of health exhibited a greater likelihood of clinical improvement, highlighting the psychological component as an integral part of treatment outcomes.
Conclusions
To summarize, this study demonstrates that a holistic therapeutic approach significantly improves the quality of life and clinical outcomes for SIBO patients, emphasizing the necessity for individualized treatments that address diet, microbiota balance, and symptom relief. Despite moderate normalization of diagnostic test results, substantial clinical improvement highlights the importance of patient-reported outcomes. This is one of the first large-scale studies to specifically evaluate quality-of-life outcomes in SIBO patients using validated tools, making it a unique contribution to the field. These findings underscore the critical need for increased awareness and acceptance among healthcare providers of comprehensive SIBO management strategies. However, the authors also acknowledge key limitations, including the lack of a placebo or control group and the difficulty in isolating the effects of individual treatment components.
Future research should investigate psychological interventions, long-term sustainability, and personalized diets to better address the profound impact of SIBO on patients' daily lives, ultimately improving care standards and patient well-being globally.
Journal reference:
- Liébana-Castillo AR, Redondo-Cuevas L, Nicolás Á, et al. Should We Treat SIBO Patients? Impact on Quality of Life and Response to Comprehensive Treatment: A Real-World Clinical Practice Study. Nutrients. (2025), DOI: 10.3390/nu17071251, https://www.mdpi.com/2072-6643/17/7/1251