How your body weight influences the interaction of plant compounds with your gut: New research reveals that rutin and genistein elicit distinct gut metabolite changes, depending on BMI, paving the way for personalized nutrition strategies.
Modulation of the human fecal metabolome – Effect of polyphenols depends on the BMI
In a recent study published in the journal Current Research in Food Science, researchers in Germany comprehensively analyzed the impacts of rutin and genistein, two plant-derived polyphenols, on the human fecal metabolome. Their findings reveal substantial differences in metabolic diversity based on the human volunteers’ body mass index (BMI), with individuals having a lower BMI demonstrating significantly more diverse metabolic responses.
Importantly, the study utilized pooled fecal samples from volunteers, which means that individual-level variability was not assessed; therefore, the findings should be considered exploratory.
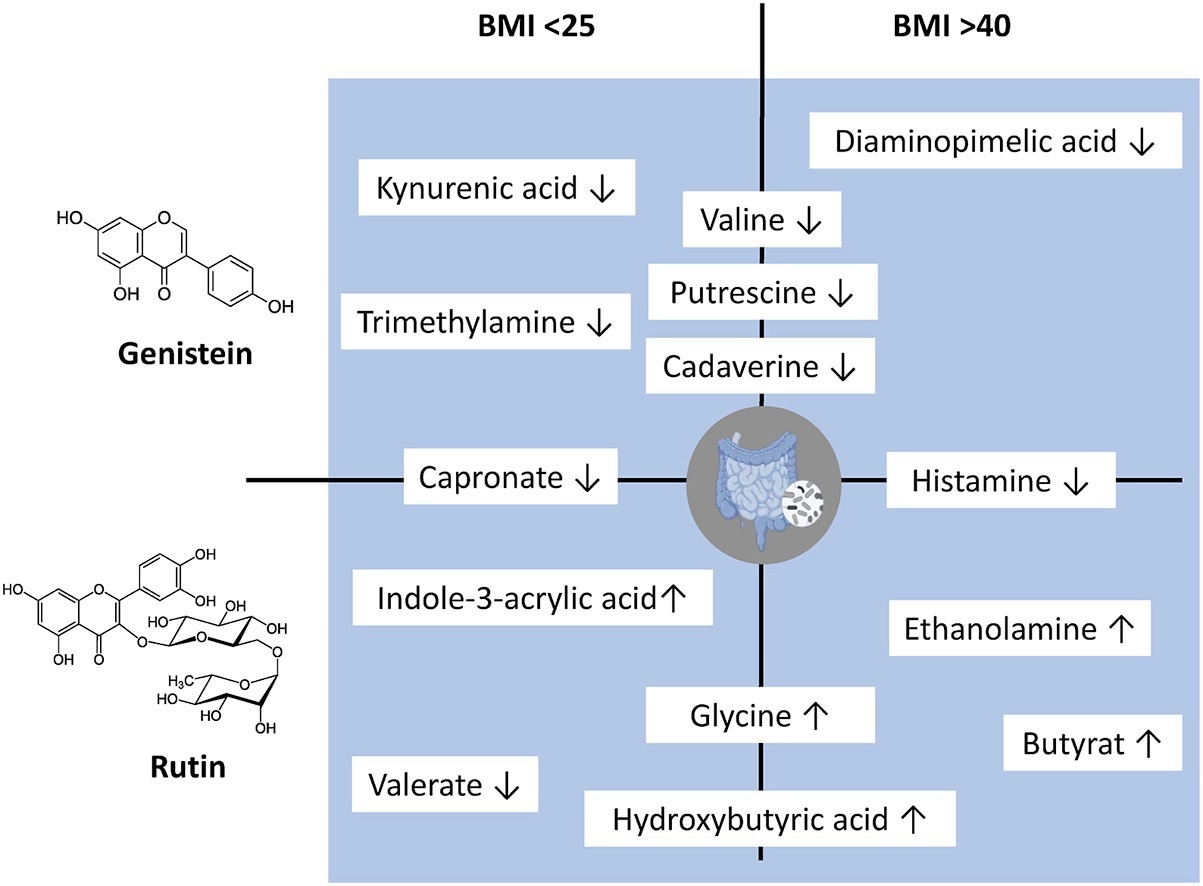
Additionally, this study highlights that rutin and genistein increase participants’ glutamine, tryptophan, and glycine levels while decreasing histamine, putrescine, cadaverine, and trimethylamine concentrations.
The researchers also observed that butyrate levels increased in the high BMI group following rutin treatment, while ethanolamine levels also rose, a change considered potentially detrimental to gut barrier integrity in obesity.
These findings offer novel insights into the interactions between polyphenols and gut microbiota metabolomes, informing future research on obesity and metabolic health. However, the authors emphasize that larger studies and dietary interventions are needed to validate these preliminary results.
Background
Obesity, a condition characterized by unwanted and unhealthy body fat accumulation, is a public health concern, with more than 1 billion adults estimated to live with the disease. It is closely associated with several metabolic and physiological diseases, including type 2 diabetes (T2D), cardiovascular diseases (CVDs), inflammatory disorders, hypertension, and even certain cancers, highlighting the need for research and policy implementations aimed at curbing its population-wide prevalence.
Recent research has revealed that obesity arises from a complex and multifaceted interplay among genetics, lifestyle, health behaviors (including diet, sleep, and exercise), and gut microbial interactions.
Studies on the gut microbiome have confirmed that individuals with obesity exhibit significantly altered gut microbial diversity compared to their counterparts with a standard body mass index (BMI), potentially promoting inflammation, appetite dysregulation, enhanced fat storage, and increased insulin resistance.
Parallel research has highlighted the importance of dietary interventions in reversing microbiome dysbiosis and promoting gut health, which in turn benefits obesity outcomes. Studies on polyphenols have shown that these plant-derived bioactives, which often escape digestion, are regularly metabolized by gut microbiota, exerting anti-inflammatory, immunomodulatory, antioxidant, anticancer, and microbiome diversity-altering effects.
Unfortunately, the mechanisms of polyphenols’ action on host physiology remain partially understood, with insufficient knowledge on their interactions with bacterial metabolic pathways and circulating metabolites.
About the study
The present study aims to address these knowledge gaps and contribute to furthering medical understanding of microbiome- and obesity-driven processes by exploring the impacts of rutin and genistein, two polyphenols with known obesity-associated benefits, on postbiotic effects. It also investigates whether host BMI (and, by extension, the degree of obesity) can influence the diversity of hosts’ fecal microbiota and the metabolites generated through the metabolism of these polyphenols.
Study samples (human fecal samples) were obtained from two volunteer cohorts: one with a low BMI (18.5–25 kg/m²; n = 7) and one with a high BMI (>40 kg/m²; n = 7). Samples were pooled within each BMI group to mask individual variability, and these samples were incubated for 48 hours under controlled conditions (10% H₂, 10% CO₂, and 80% N₂; temperature = 37°C) with high-purity (≥98%) rutin and genistein (Cayman Chemical, Estonia) in triplicate. Following incubation, an adapter-based SIMPLEX protocol was used to extract both hydrophilic and lipophilic metabolites for characterization and subsequent downstream analyses.
Characterization was achieved using a direct injection Fourier-transform ion cyclotron resonance mass spectrometer (DI-FT-ICR-MS) and tandem high-performance liquid chromatography (HPLC). Resultant chromatograms were analyzed using the MetaboScape (2021b) platform, with subsequent annotation leveraging the Human Metabolome Database (HMDB 5.0) annotation database.
The HMDB database was also used to elucidate metabolite grouping and pathway enrichment. Finally, the MetaboAnalyst 5.0 platform was used to evaluate the differences between metabolite profiles of individuals with high BMI, low BMI, and control BMI.
Study findings
The study identified 361 metabolites (35 groups) that were significantly regulated (i.e., concentrations higher or lower than corresponding controls) by rutin and genistein. Notably, fecal material from low BMI volunteers demonstrated responses from more substance classes (more metabolites and groups; n = 88 metabolites) than high BMI fecal samples (n = 45 metabolites). 16S rRNA gene metagenomics confirmed these findings by revealing a higher α-diversity in lower BMI fecal matter than in higher BMI fecal matter post-incubation.
“…a higher diversity in the response may be associated with a higher diversity of the microbial composition. It is known that there is a decrease in gut microbial diversity in the context of obesity, as was first described by Turnbaugh et al. Nicholson et al. postulated that the state or condition of the intestinal microbiome is reflected in human biofluids such as serum and can be determined by metabolomics.”
Rutin and genistein were observed to alter concentrations of amino acids, peptides, and their analogs the most, followed by carbohydrates and carbohydrate derivatives. Notably, several identified regulated metabolites have been implicated in prior research on chronic diseases, highlighting the complex interplay between dietary polyphenols, microbial diversity, and host health.
Furthermore, while some metabolites demonstrated beneficial regulation (e.g., increased butyrate levels and reduced lactate levels in high BMI samples following rutin treatment), others (e.g., increased ethanolamine levels in the high BMI group) were found to be detrimental in the context of obesity.
“This suggests the possibility that the application of polyphenols may not be equally suitable for everyone and thus may not lead to the same results or potential beneficial effects for all individuals, emphasizing the idea of personalized nutrition. Therefore, results of the explorative study would require validation with a larger sample size, biological replicates, and/or an intervention study.”
Conclusions
The present study provides novel insights into the mechanisms governing the interactions between dietary polyphenols, gut microbial health, and host health. It demonstrates that these interactions are not unidirectional but rather bidirectional—BMI can alter gut microbial profiles, thereby altering polyphenol metabolism, which, in turn, impacts BMI. Moreover, specific BMI-dependent differences were noted, such as beneficial changes (e.g., higher butyrate and glycine) and potentially adverse ones (e.g., higher ethanolamine), highlighting the complexity of host-microbe-polyphenol interactions. It also provides a methodological approach for future research to investigate the impacts of plant compounds, dietary components, and drugs on gut microbiota and metabolomic profiles.
However, the authors caution that, due to the small sample size, pooling of samples, and exploratory design, these results should be interpreted with caution and confirmed in larger, well-controlled human studies.
Journal reference:
- Jensen-Kroll, J., Demetrowitsch, T., Sprotte, S., Brix, F., Beckmann, A., Schlicht, K., Laudes, M., Hasler, M., Franz, C. M. A. P., & Schwarz, K. (2025). Modulation of the human fecal metabolome – Effect of polyphenols depends on the BMI. In Current Research in Food Science (p. 101060). Elsevier BV, DOI: 10.1016/j.crfs.2025.101060, https://www.sciencedirect.com/science/article/pii/S2665927125000917