Introduction
Protein structure and function can be accurately studied using intrinsic fluorescence measurements. A better understanding about the activity or conformational states of proteins under various temperatures, pH, and ion concentrations can be achieved by estimating the quantity of fluorescence.
A reliable excitation source is a key requirement for measuring fluorescence. A cost-effective solution for this is high-power UV LEDs that help obtain key data regarding amino acids and proteins.
Instruments
To demonstrate this technique, a 280nm UV LED was used along with a back-thinned CCD array spectrometer to determine the fluorescence of samples of bovine serum albumin (BSA), a protein critical to the body’s biochemical processes, and lysozyme, a natural enzyme commonly used as a bacterial agent, in different conformational states.
The demonstration revealed that molecular spectroscopy is a powerful approach for measuring protein fluorescence. Spectroscopy has been proven to be flexible enough to be used along with biological as well as non-biological samples that fluoresce upon UV excitation.
Fluorescence Spectrum
Aromatic amino acids, present in most proteins, fluoresce upon UV excitation. The fluorescence spectrum of a protein gives a clear idea of the conformational state of the protein and its amino acid composition.
During the transition of the protein from folded to unfolded states, the local environment of the aromatic amino acids also changes, which in turn affects their fluorescence properties.
Protein unfolding can be monitored using these changes in their intrinsic fluorescence. This information is extremely useful in medical diagnostics, where neurodegenerative diseases related to improper protein unfolding are being investigated.
The protein’s native state can be modified using chaotropic or other chemical agents such as guanidine hydrochloride or urea, high temperature, or pH changes. Once the protein starts unfolding, amino acids embedded within the protein’s hydrophobic core come in contact with the solvent. Solvent exposure causes susceptibility to quenching agents and thus reduces the fluorescence of tyrosine, tryptophan, and phenylalanine.
The fluorescence from BSA and lysozyme samples was measured using a 280nm UV LED with a high-sensitivity spectrometer. In order to demonstrate the effect of protein conformation, the fluorescence spectra of the proteins in a phosphate buffered saline (1X PBS pH 7.4) and 0.1 M HCl/KCl (pH 1) solution were quantified.
Measurement Conditions
Samples of 12mg/mL BSA (A2153 Sigma) and 3mg/mL lysozyme (L6876 Sigma) in 1X PBS and 0.1 M HCl/KCl were prepared. PBS functions as a buffer solution that helps maintain the pH of the sample. Protein suspended in the lower pH 0.1 M HCl/KCl solution begins to unfold and exposes its amino acids to the solvent.
In order to measure the optimal excitation wavelength of tryptophan and use it as a reference for tryptophan fluorescence, a solution of 5mg/mL L-Tryptophan (T90204 Aldrich) in deionized water was prepared. Both absorbance and fluorescence measurements were carried out.
Results and Discussion
Tryptophan shows the highest quantum yield of aromatic amino acids in the fluorescence spectra. Its absorbance spectrum shows a peak at ~280nm originating from its indole group. The absorption spectrum of tyrosine is similar to that of tryptophan and has optimal excitation near 274nm.
These absorbance properties clearly illustrate that a 280nm UV LED can be employed to excite tryptophan and tyrosine for monitoring protein unfolding.
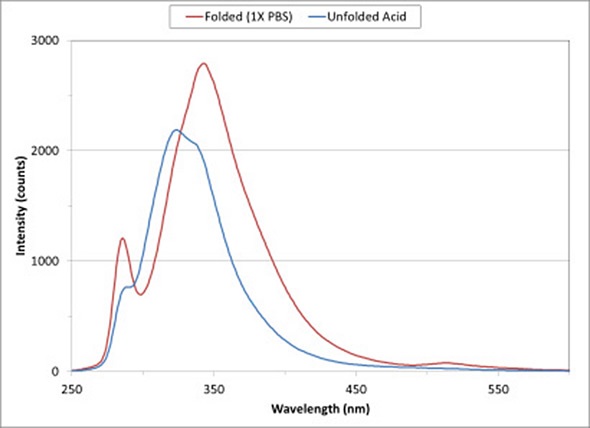
Figure 1 Fluorescence spectra of BSA show changes in protein structure on exposure to a low-pH buffer.
Figure 1 shows the fluorescence spectra for a solution of BSA in 1X PBS and 0.1 M HCl/KCl. As a major constituent of blood plasma, BSA acts as a carrier protein and regulates osmotic pressure to aid the right distribution of body fluids.
On exposure to 0.1 M HCl/KCl solution, the conformational state of the protein changes to expose the tryptophan and tyrosine amino acids to the solvent. As the protein moves from a folded state in 1X PBS (indicated by the red curve) to the unfolded state (indicated by the blue curve), the intensity of the fluorescence spectrum decreases and peak fluorescence moves to a shorter wavelength.
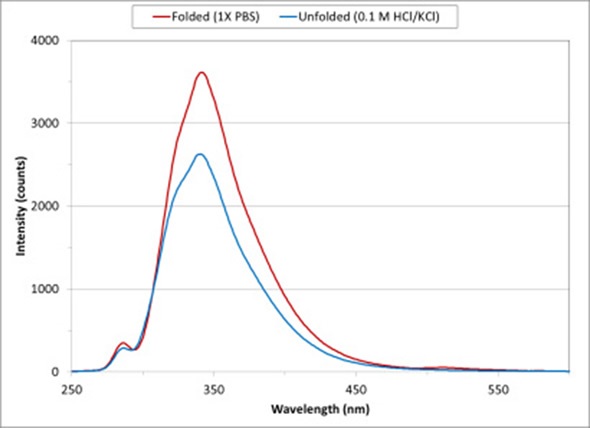
Figure 2 Lysozyme protein conformation change from folded to unfolded state on exposure to low pH solution
Figure 2 shows the intrinsic fluorescence spectra for lysozyme diluted using 1X PBS and 0.1 M HCl/KCl. Lysozyme is a powerful enzyme that breaks down the bacterial cell wall. Lysozyme from chicken egg whites has been used for these measurements.
On exposure to a low pH solution, the conformational states of the protein changes and exposes tryptophan and tyrosine amino acids to the solvent. As the protein moves from a folded conformation in 1X PBS (represented by the red curve) to an unfolded state (represented by the blue curve), the fluorescence spectrum decreases in intensity.
Conclusion
The results of fluorescence measurements illustrated above for lysozyme and BSA give insights into a larger picture of the conformation of these proteins. High concentrations of chaotropic agents such as DMSO or guanidine can be used to obtain more granular data about the protein’s conformational states. Steady increase in pH or temperature could also aid in tracking the unfolding process with fluorescence.
Powerful LED excitation sources in a wavelength range of 240 to 627nm offer innumerable opportunities to study intrinsic fluorescence of proteins. The broad wavelength range allows testing with many excitation wavelengths, enabling researchers to employ the optimal excitation wavelength for every fluorophore. UV LEDs integrated with a flexible sampling system and a configurable spectrometer allow a wide range of fluorescence and absorbance measurements.
Acknowledgment
Produced from content authored by Yvette Mattley, PhD.
Reference
http://www.physics.nus.edu.sg/~Biophysics/pc3267/Fluorescence%20Spectroscopy2007.pdf
About Ocean Optics
 Ocean Optics is a diversified photonics technology firm and a global leader in optical sensing. With full-service locations in the United States, Europe and Asia, we serve a wide range of markets, including process control, consumer electronics and medical diagnostics.
Ocean Optics is a diversified photonics technology firm and a global leader in optical sensing. With full-service locations in the United States, Europe and Asia, we serve a wide range of markets, including process control, consumer electronics and medical diagnostics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.