Brief history of penicillin
1896 - Ernest Duchesne observed antibacterial properties of mold.
1928 – While studying staphylococci, Alexander Fleming observed a culture died after being contaminated with mould and attributed this action to penicillin.
1930 - Initial testing of drugs was carried out on selected patients.
1940s - Merck & Co. penicillin was being mass produced for war.
1945 - Bacterial resistance began to be studied.

Penicillin activity
Penicillin can weaken bacterial cell walls, and therefore it is an active antibiotic. The cell wall is made up of alternating linkages of N-acetylglucosamine and N-acetylmuramic acid.
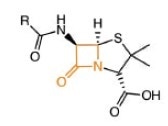
Penicillin core
Tetrapeptides dangling in the muramic acid linkages consisting of D-alanine, L-alanine, L-lysine and D-glucosamine can create cross-links between rows.
Crosslinking is prevented by β-lactam antibiotics, leaving the bacterial cells susceptible to attack.1, 2 But a naturally occurring enzyme β-lactamase can hydrolyze the β-lactam ring, rendering it ineffective.
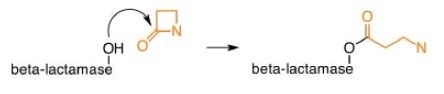
Penicillin development
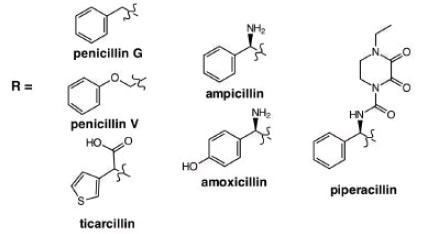
1. Derivatization - alter sterics, bioavailability, etc.
2. ß-lactamase inhibitors - irreversibly bind to ß-lactamase
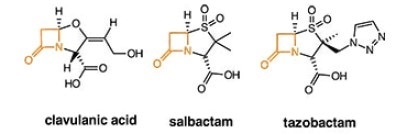
Composite penem antibiotics
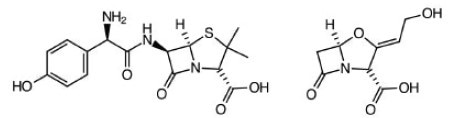
Amoxicillin & Clavulate. Routine ear, lung and sinus infections. Highest bioavailability of penicillin drugs.
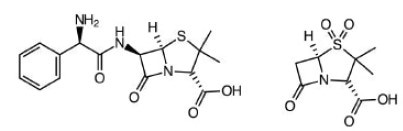
Ampicillin & Salbactam. Skin, bone, gynecological and abdominal infections. Typically, second line defense when bacteria found to be resistant to other derivatives
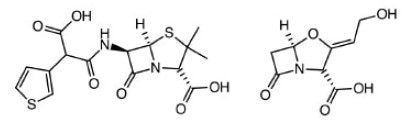
Ticarcillin & Clavulate. Blood, bone, respiratory, urinary tract infections.
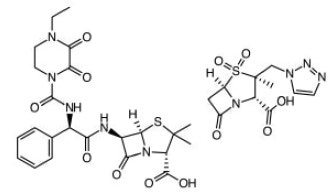
Piperacillin & Tazobactam. Most active antibiotic against Klebisella bacteria in pneumonia, Urinary tract infections (UTIs), meningitis, blood diseases etc.
Example 1H NMR data from penicillin family
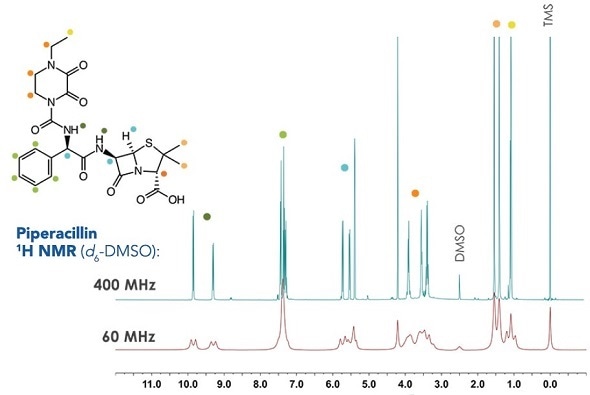
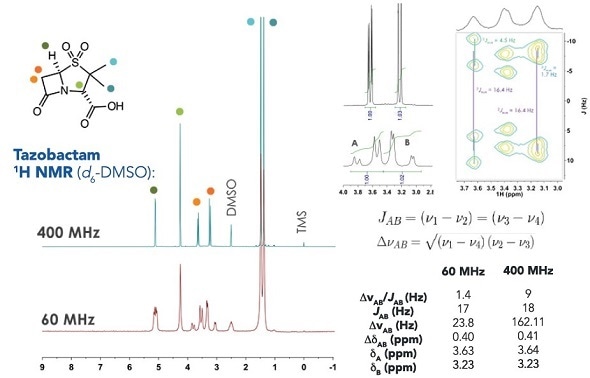
Structural confirmation software tools
In spite of second order effects, several software packages are available to validate the structure and detect impurities (such as ACD/Labs Spectrus Processor, Mnova Verify, or Perch Solutions, as illustrated here where the red trace is simulated, the blue trace is experimental, and the green trace is the difference).
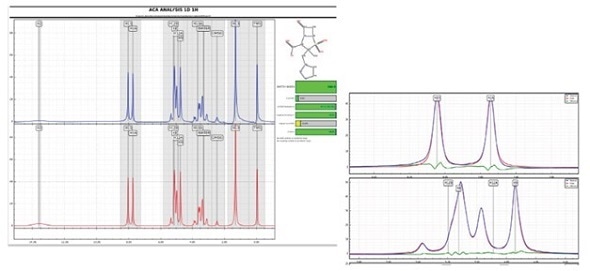
Courtesy of Perch Solutions at perchsolutions.com
Relative compositional analysis
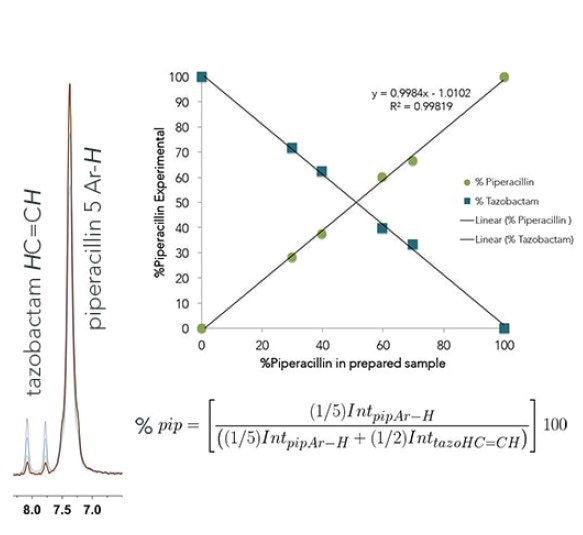
Shown below are overlaid 1H NMR spectra of piperacillin-tazobactam mixtures of different relative concentrations for use in both automated and manual quantification.
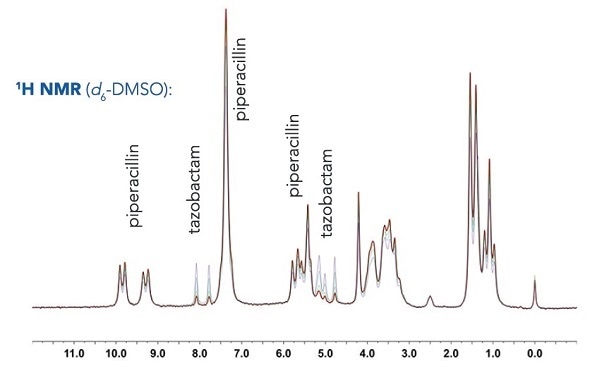

Manual integration
Even though there is overlap of the 1H resonances of tazobactam and piperacillin, distinctive regions can be seen and these can be incorporated for each component. Here, the 2 CH=CH peaks have been selected from the tazobactam triazole ring (δ = 7.6595-8.2236 ppm) and the 5 aromatic CH's have been chosen from the monosubstituted benzene ring in piperacillin (δ = 6.8856-7.6595 ppm).
The percent piperacillin in a d6-DMSO solution can be determined using the normalized integral of each region. This was carried out for a known concentration series (0,30, 40, 60, 70, and 100% piperacillin) and also with an unknown injection mixture to find out the linearity and accuracy of this qNMR experiment.
The results revealed that the injection mixture contained 83.11% piperacillin and 16.88% tazobactam. This value agrees well with the known ratio in the prepared injection mixture of 82.3:17.7%.
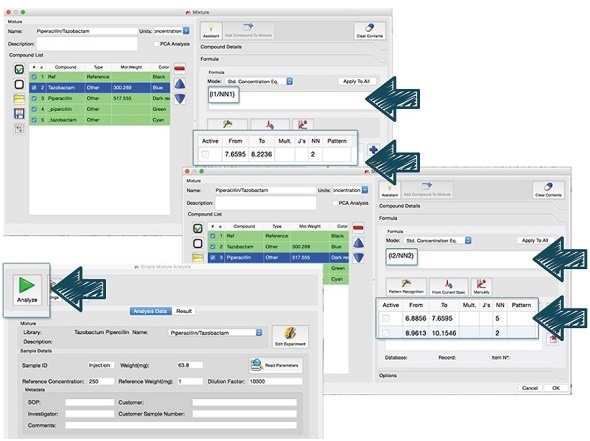
Automated analysis
Mnova's Simple Mixture Analysis (SMA) was used to determine the piperacillin to tazobactam ratio in order to facilitate the routine qNMR analysis. This approach enables a user to expedite the analysis and make it easy for non-experts.
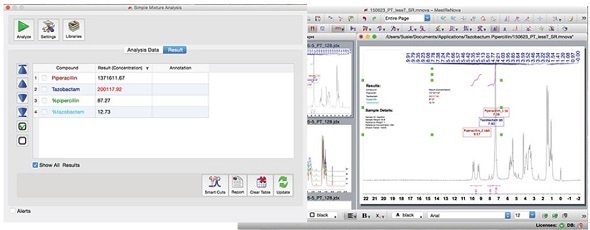
After establishing the method, the concentration or relative percentage can be easily determined by anyone by simply clicking on the green 'Analyze' button.
References and Further Reading
- Lewis, K. Nature Rev Drug Disc, 2013,12,371.
- Aminov, R. I. Front. Microbiol, 2010, 134,1.
About Nanalysis

Nanalysis was established in 2009. The company develops and manufactures portable Nuclear Magnetic Resonance (NMR) spectrometers for the laboratory instrumentation market.
In the fall of 2012, the first product was launched: NMReady™, the first fully featured portable NMR spectrometer in a single compact enclosure requiring no liquid helium or any other cryogens.
The NMReady is used by chemical professionals in all types of industries (oil & gas, chemical, pharma, biotech, food processing) as well as government and university labs.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.