Introduction
Accurate test results depend on the integrity of urine strips. Irrespective of brand, improper handling of strips can result in false results and thus lead to possible incorrect diagnosis. Strip bottles that are not tightened or recapped properly will expose the contents to humidity in room air, which could affect strip integrity, cause reagent degradation and ultimately lead to false results.
Crolla et al.1 performed a study, in which three manufacturer’s instruments and reagent strips were compared after exposing the test strips to room air. Strip containers should be closed after use as recommended by the manufacturer, or else this will lead to room air exposure. This article reports the study results, which compare the MULTISTIX® 10SG urine strips and the Siemens CLINITEK Status®+ Analyzer with offerings from two other manufacturers.
Auto-Checks—improving clinical information and urinalysis workflow
The Siemens MULTISTIX® series of urine reagent strips (Figure 1) feature new identification (ID) bands that allow a series of automatic quality checks (Auto-Checks) when used in combination with the CLINITEK Status range⒜ of urine chemistry analyzers shown in Figure 2.
Proprietary Auto-Checks technology automatically:
- Identifies the Siemens reagent strip type—removing manual data entry and saving time
- Checks for common sample interferences⒞ (high pH, high specific gravity, visibly bloody urine, elevated glucose)—the analyzer alerts to these interfering conditions and documents if results may be impacted.
- Detects the humidity overexposure of test strips⒝—the analyzer reduces false-positive results by not reporting the results for this condition

Figure 1. MULTISTIX 10 SG reagent strips with ID bands.

Figure 2. CLINITEK Status family of analyzers employs an algorithm for humidity-compromised reagent strip detection that helps ensure quality results.
- CLINITEK Status® Connect and CLINITEK Status® legacy analyzers upgraded to version 2.0 or greater.
- Only available with test strips that have the leukocyte pad.
- Feature not available in the U.S.
Materials and methods
The Crolla et al. study evaluated results generated by three manufacturers’ reagent strip and analyzer combinations:
- Clarity® UROCHECK strips read on the Urocheck 120 Analyzer (Diagnostic Test Group)
- MULTISTIX 10 SG strips read on the CLINITEK Status+ Analyzer (Siemens Healthcare Diagnostics)
- CHEMSTRIP® 10 MD strips read on the Urisys 1100® Analyzer (Roche Diagnostics)
For each manufacturer, two sets of reagent strips were prepared. The first set of bottles were opened and left exposed for 40-plus days to room air (22oC to 26oC) and room humidity (26% to 56%). This is done to simulate the exposure the reagent strips could receive when an operator does not correctly close the strip container (stressed strips). In the second set, the bottles were left sealed until the urine sample was tested (unstressed strips).
About 200 patient urine samples were tested across all three combinations of brands. Errors or inadequate volumes that happened during testing cause the samples to differ slightly. The total number of samples tested by the manufacturer is detailed in Table 1. Reagent strips were tested with patient samples for the below given analytes:
- leukocytes
- occult blood
- protein
- nitrate
- ketone
- bilirubin
- urobilinogen
- pH
- specific gravity
- glucose
The testing of the urine samples was done over three months. The samples were tested across all instrument systems in duplicate for each set of strips, stressed and unstressed. These duplicate samples were run consecutively for each combination of strips and analyzers.
An outpatient treatment center located in an urban area was the study setting. Most of the testing was completed by medical assistants and nursing personnel with an intermittent test being performed by a trained (ASCP) laboratorian.
This combination of operators replicated the exact testing conditions in the treatment center. All operators were trained, and their competency assessed on all three analyzers before data was collected.
Results and discussion
In the study performed by Crolla et al., the analyte performance agreement between unstressed and stressed reagent strips was first evaluated by checking the first replicate of each tested set, followed by comparing this agreement with the agreement between the results obtained from the unstressed (control) – replicate 1 and replicate 2.
MULTISTIX 10 SG strips, which are read by the CLINITEK Status+ Analyzer, are designed to return an error flag instead of an actual result as soon as the system detects that the strips have been potentially affected because of excess exposure to environmental humidity.
When tested on the CLINITEK Status+ Analyzer, over 95% (95% confidence interval: 95.9% to 99.7%) of the stressed MULTISTIX 10 SG strips returned error flags which accurately indicated that the reagent strips had been affected and hence are not suitable for use (Table 1).
Table 1. Error-flagged results for unstressed and stressed (humidity compromised) test strips, by manufacturer
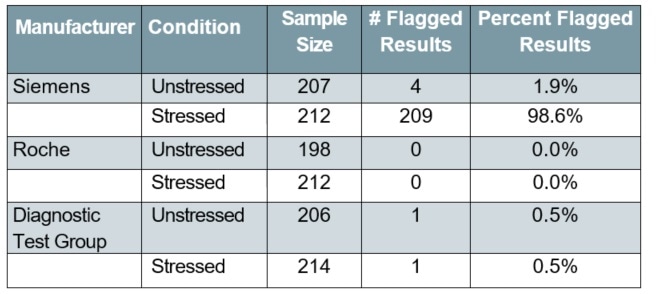
For environmentally stressed MULTISTIX 10 SG strips, the flagged sample rate was 98.6%. As a result, all further analysis of stressed performance in this work included only those reagent strips that were developed by Diagnostic Test Group and Roche, because the Siemens strips failed to give performance data post-stress.
The percent agreement (both exact and ±1 group) between two replicates of unstressed reagent strips for all three manufacturers’ materials was the performance of the unstressed strips (control condition). A ±1 block scale was employed by the authors, as this is the usual acceptable variance for urine strips.
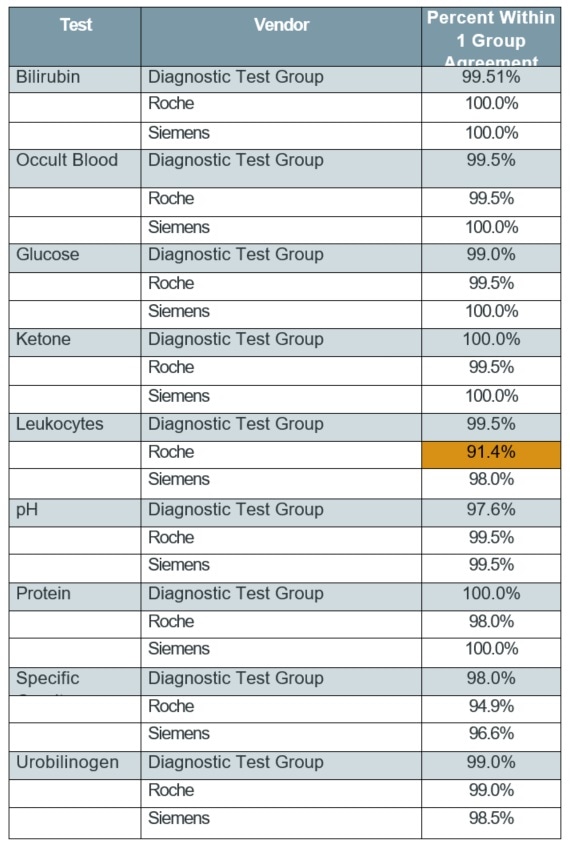
Tables 2 and 3 show the summarized results, which reveal no major difference (p>0.05) in replicate agreement among the three manufacturers’ reagent strips in an unstressed condition, using either exact or ±1 block scale.
Depending on replicate agreement rates for other manufacturer’ unstressed strips, it was observed that there were just two instances of considerably different % agreements for the two replicates of unstressed reagent strips. These instances are highlighted.
Table 2. Unstressed strips—replicate agreement (exact)
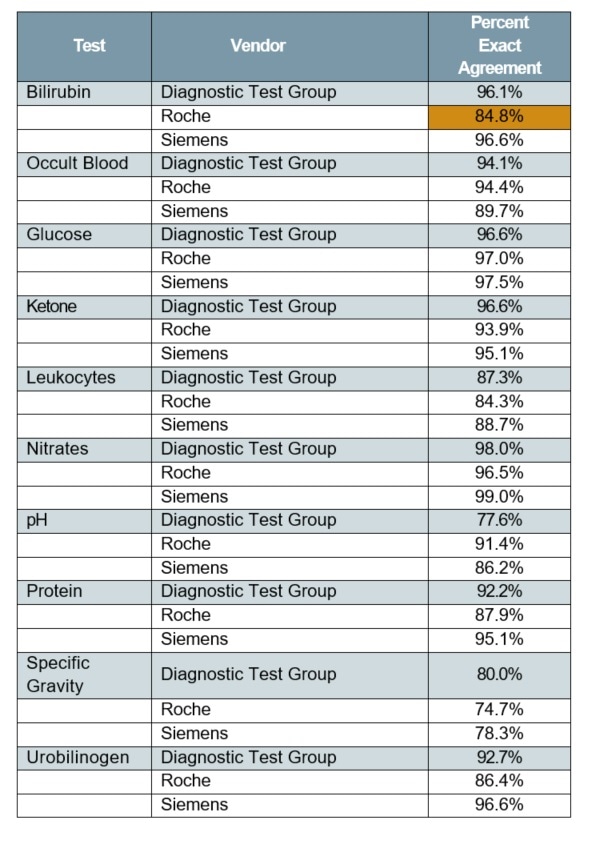
Table 3. Unstressed strips—replicate agreement (±1 group)
For Roche and Diagnostic Test Group, the percent agreement between the first replicate of the stressed strip and the first replicate of the unstressed strip were determined to assess the performance of environmentally stressed reagent strips.
Tables 4 and 5 summarize the results for each of the analytes. Those analytes were the percent agreement for the stressed conditions varies considerably from the percent agreement for the control conditions are flagged as “Significant” (p<0.05) in these tables.
Table 4. Performance of stressed vs. unstressed strips (exact)
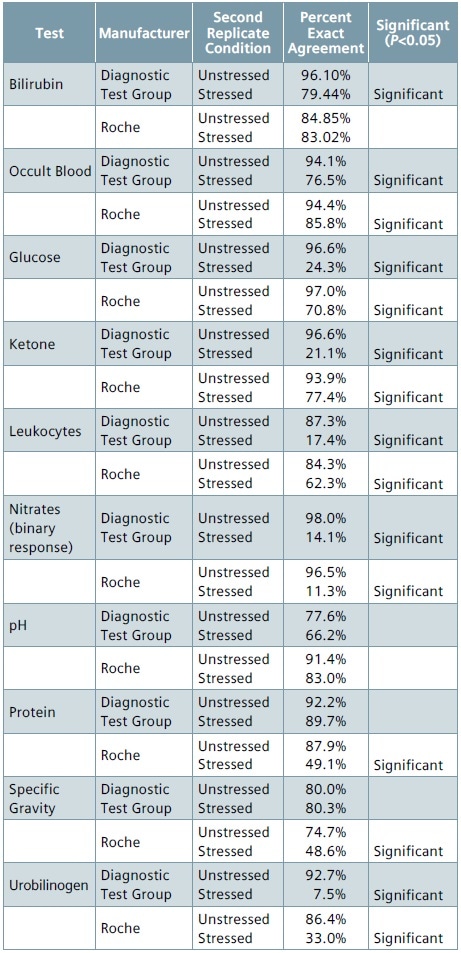
Table 5. Performance of stressed vs. unstressed strips (±1 group)
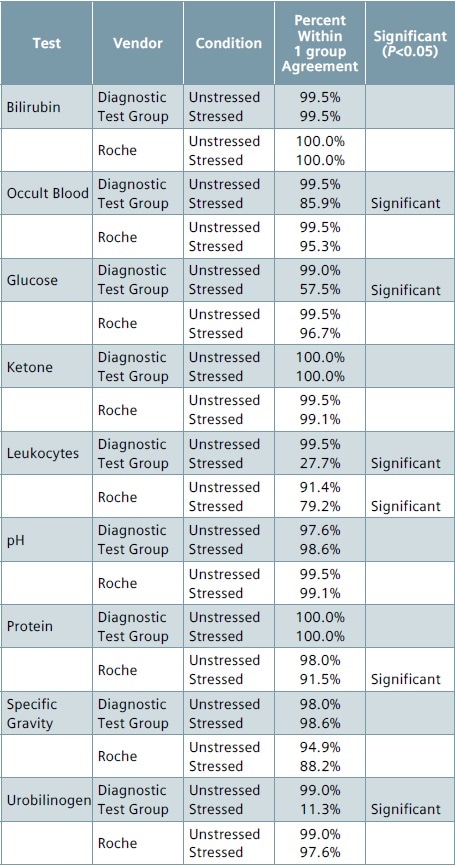
As nitrate tests return a binary (negative/positive) result, they are considered a candidate for analysis using the ±1 group criterion. With regards to nitrate, the stressed strips from Diagnostic Test Group and Roche had just 11.3% to 14.1% agreement between the nitrate results obtained from replicate 1 of the unstressed condition and replicate 1 of the stressed condition when compared to 96.5% to 98% agreement observed between the replicates of unstressed condition (control).
For numeric or non-binary analyte responses, the highest percentage of disagreement between the stressed and unstressed strips for exact block output was seen with ketone, glucose, urobilinogen and leukocyte tests on Roche as well as Diagnostic Test Group strips.
On expanding the agreement criterion to ±1 group, the disagreement was considerably reduced for the Roche strips, except for protein (91.5% agreement) and leukocyte (79.2% agreement), and with both agreement rates being considerably different from the unstressed (control) agreement.
In the case of Diagnostic Test Group strips, the percentage agreement for urobilinogen (11.3%), leukocytes (27.7%), and glucose (57.5%) continued to be considerably lower when compared to their respective unstressed conditions.
On the basis of the data obtained for the Roche and Diagnostic Test Group reagent strip and analyzer combinations, major differences were observed between the unstressed and stressed results as a result of exposure to humidity and room air. Therefore, an inaccurate diagnosis and treatment may occur based on the wrong results produced from the exposed strips.
An automatic warning mechanism in the Siemens analyzer prevents the results from being reported on detection of humidity exposure. In the controlled study, this analyzer would have prevented erroneous reporting and produced an error message rather than produce a result.

Conclusion
The CLINITEK Status+ Analyzer and Siemens MULTISTIX 10 SG urinalysis strips are integrated with Auto-Checks technology that enables automatic detection of reagent strips that could have been affected by too much humidity.
The CLINITEK Status+ Analyzer not only detected MULTISTIX 10 SG strips that had been affected by excess humidity, but also prevented possibly inaccurate results from being reported.
The Roche and Diagnostic Test Group analyzers do not feature a humidity detection system. Although the test strips were being affected by excess humidity, these two instruments reported results for patient samples. The reported results could be erroneous because analyte results varied between unexposed (unstressed) and exposed (stressed) test strips even for the same patient sample.
During various assessments of laboratories, Crolla and team had observed that the cover in the urine-strip bottles is partly or fully removed most of the time. The analysis highlights the necessity for testing entities so that the recommendation of individual manufacturer can be strongly enforced to keep strip containers capped when strips are not being taken off for further analysis.
It would also be equally beneficial in cases where there are many operators, which makes it rather complicated to establish compliance, to employ a system that would inform the tester about a certain affected strip and thus not enable testing.
Acknowledgements
Produced from materials originally authored by Lawrence Crolla, Cindy Jiménez and Pallavi Patel from the Northwest Community Hospital, Arlington Heights, IL.
References
- Crolla L, Jimenez C, Patel P. Evaluation of an automated humidity check for instrument-read urinalysis strips. MLO Online. 2011. Online: https://www.mlo-online.com/home/article/13004210/evaluation-of-an-automated-humidity-check-for-instrument-read-urinalysis-strips
- Gallagher EJ, Schwartz E, Weinstein RS. Performance characteristics of urine dipsticks stored in open containers. Am J Emerg Med. 1990; 8:121-123.
- Little P, Moore MV, Turner S, et al. Effectiveness of five different approaches in management of urinary tract infection: randomized controlled trial. BMJ. 2010; 340:c199.
- Propp DA, Weber D, Ciesla ML. Reliability of a urine dipstick in emergency department patients. Ann Emerg Med. 1989; 18:560-563.
- Shulkin DJ, DeTore AW, Ringenberg BJ, et al. When laboratory tests are abnormal and the patient feels fine—Dipstick test in the ED Performance characteristics of urine dipsticks stored in open containers. Hosp Pract (Off Ed). 1990; 25:85-86, 89-90, 92.
About Siemens Healthineers Point of Care Diagnostics

Point-of-care solutions are designed to provide immediate, convenient, and easy-to-use diagnostic testing. From the ED to the physician’s office, clinical management decisions can be made immediately and result in improved patient safety, clinical outcomes, and overall patient satisfaction.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.