Cardiovascular interventional therapy with stents has emerged as the most effective method to treat coronary heart disease. However, thrombosis and hyperplasia remain the usual pathological responses to the implantation of foreign devices. Originally stents were made of steel, but recently these have been superseded by the polymer. Modern developments have introduced a new range of stents made from bio-resorbable polymers but problems such as thrombosis and hyperplasia still exist.
In order to suppress this immune response as well as overgrowth and subsequent restenosis, anti-inflammatory drugs are loaded onto the surface of stent implants.
Kratos have investigated the surface of drug loaded polymer stents. These stents were made of polylactic acid (PLA) dosed with an anti-inflammatory drug with a molecular structure of C51HxNO13. XPS yields quantitative information regarding drug distribution and using Argon cluster sputtering the distribution of the drug into the stent structure can be seen. Analysis was also performed on stents submerged in buffer solution (PBS) to see the effects on aging and the propensity for the drug to migrate into the solution with time.
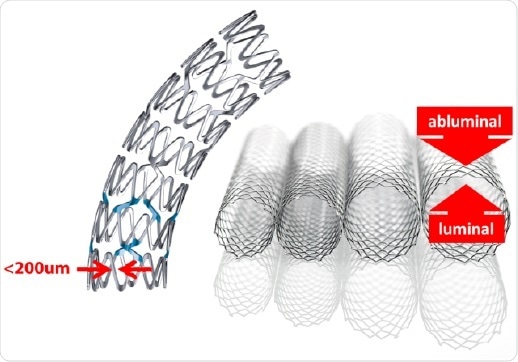
Figure 1. Examples of stent architecture.
Experimental
Using the state-of-the-art AXIS Supra spectrometer equipped with the gas cluster ion source (GCIS), XPS was performed. It should be noted that etch rates were calibrated with PLA thin-film standards.
Results
The polymer stents were introduced into the analysis chamber spectra were acquired on the luminal (inner) and abluminal (outer) surfaces. The narrow nature of the stent scaffold structure are typically less than 200 µm and small-spot spectroscopy was employed with an analysis area of 110 µm. These surfaces were analyzed in three spots to evaluate evenness of coverage. The stent polymer and drug chemical structure consist of carbon and oxygen atoms with the differentiating marker atom used to evaluate drug concentration being Nitrogen. This showed that the average concentration is significantly higher on the abluminal surface which is consistent with the manufacturing process.
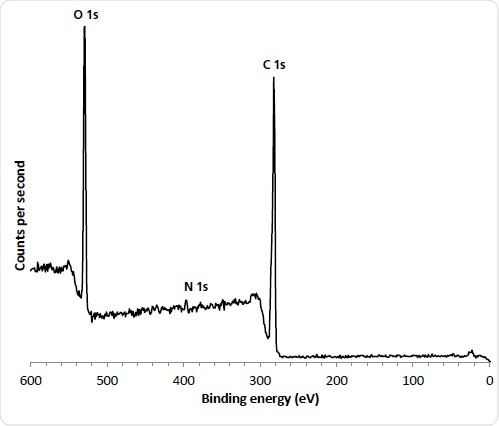
Figure 2. 110 µm small spot spectrum from ‘as received’ abluminal stent surface.
Conventional depth profiling techniques were applied using Argon cluster ions, Arn+ to investigate the distribution of the drug from the outermost surface into the stent. The stent surface was etched with cluster ions to remove the upper layer before it was reanalyzed. For this particular depth profile, 10 kV Ar1000+ ions were used. This is because this etch mode allows for rapid etching and reduces experiment time, while retaining the chemical structure of the drug/polymer materials.
It should also be noted that conventional methods to study the effects of aging and drug mobility of stents involve immersion in buffer solution for varying time intervals. The results in the solution being analyzed with HPLC to see the extent of drug dissolution from the stent surface.
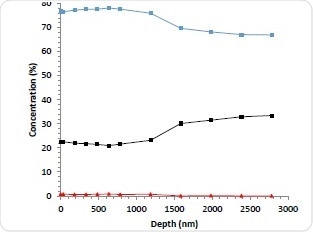
Figure 3a. 10 kV Ar1000+ depth profile of abluminal surface; Carbon (blue), Oxygen (black) Nitrogen (red).
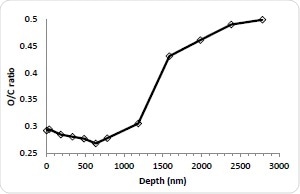
Figure 3b. C/O ratio.
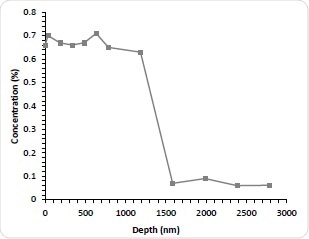
Figure 3c. Nitrogen concentration as a function of etch time.
Although this method is thought to be accurate in determining the amount of drug that has departed from the stent, it is still unknown how much remains and how it is distributed. It is therefore deemed appropriate to explore this by sputter depth profiling. The stents were immersed for 1-3 months (1M, 2M, and 3M) in PBS. They were then dried, reanalyzed and depth profiled. As expected, all stents immersed in PBS demonstrated a significant decrease in the N concentration is indicating drug dissolution after the first etch cycle probably due to the removal of weakly absorbed N-containing contamination. The Nitrogen concentration of subsequent cycles was less than 0.1 atomic %. Astonishingly, after 100 nm deep into the surface, the concentration of Nitrogen begins to rise again for all three aged stents. This indicates that despite significant depletion of the drug in the near surface region there is still a remaining fraction of the bulk. In addition to this, a similar profile shape to the as-received stent can then be seen with further etching with the Nitrogen concentration peaking and decreasing with depth. By increasing the PBS exposure, the Nitrogen maximum decreases. For the immersed stents the interface between the drug layer and the stent bulk is not as sharp which is most likely due to pitting and corrosion of the stent structure. This is known to be a well-documented effect of solution exposure. As would be expected, the integral of the Nitrogen concentration with depth decreases with longer PBS exposure and the results of this is thought to be useful for future kinetic studies into the dissolution mechanism.
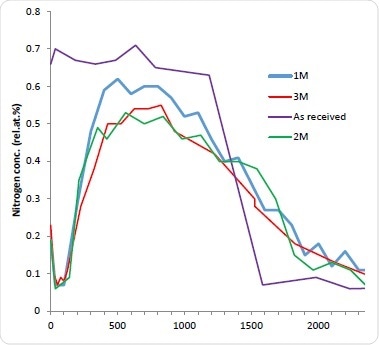
Figure 4. 10 kV Ar1000+ depth profiles of stents immersed in PBS for ‘as received’ (purple) 1M (blue) 2M (green) and 3M (red).
Conclusion
In conclusion, the results show that using a small area XPS and Arn+ cluster depth profiling to investigate the distribution of anti-inflammatory drugs coated on the surface of bio resorbable stents shows that the abluminal surface of the as manufactured stent was fully characterized for drug concentration, layer thickness and drug distribution.
Enabling technology by allowing XPS analysis of novel biomaterial devices such as the AXIS Supra (XPS) surface analysis instrument by Kratos. The use of cluster profiling allows delicate polymer materials to be analyzed with confidence.
Table 1. Surface analysis of aluminal and luminal stent surfaces.
| SPOT |
ELEMENT |
ATOMIC CONC. [%] |
| Abluminal |
Luminal |
| 1 |
C 1s |
74.78 |
73.17 |
| N 1s |
0.84 |
0.73 |
| O 1s |
24.2 |
25.33 |
| 2 |
O 1s |
23.48 |
25.42 |
| N 1s |
0.79 |
0.65 |
| C 1s |
75.69 |
73.77 |
| 3 |
O 1s |
25.34 |
22.07 |
| N 1s |
0.83 |
0.41 |
| C 1s |
73.3 |
76.16 |
| mean N conc. |
0.82 |
0.59 |
| drug conc. % |
52.5 |
37.8 |
About Kratos Analytical, Ltd.
 For the last 46 years Kratos Analytical has provided state-of-the-art spectrometers for surface analysis and we are committed to continuing with development of leading technologies. As we approach our fifth decade Kratos Analytical is still dedicated to providing service, quality and customer satisfaction, and we believe that this specialist web area will help you find the information you need for your surface analytical applications.
For the last 46 years Kratos Analytical has provided state-of-the-art spectrometers for surface analysis and we are committed to continuing with development of leading technologies. As we approach our fifth decade Kratos Analytical is still dedicated to providing service, quality and customer satisfaction, and we believe that this specialist web area will help you find the information you need for your surface analytical applications.
Global Headquarters
Kratos Analytical has its headquaters in Manchester, UK and offices in the USA, Germany and Japan. We have a large network of agents and representatives throughout the world.
The Kratos Analytical Ltd is based in Manchester, UK, and prides itself on being highly innovative, responsive, and internationally focused. Its strengths lie in the quality of design, in the excellence of its products and in the commitment to partnerships with customers. This market-leading technology is backed by the skills and experience of a responsive, forward-looking workforce.
Shimadzu Corporation
Kratos Analytical Limited is a wholly owned subsidiary of the Shimadzu Corporation of Kyoto, Japan. Shimadzu Corporation is a multi-billion dollar supplier of a wide range of scientific and industrial products to world markets.
Globally the Shimadzu corporation employs 7000 staff, it has a growing number of manufacturing facilities world wide including USA, The People’s Republic of China, Australia, the Philippines and the UK with sales and distribution outlets in almost all countries in the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.