Aroma products generally contain ethyl crotonate, trans-(ethyl but-2-enoate) as one of their components. A clear, colorless liquid with a pungent odor, this organic compound is highly flammable and does not dissolve in water. A solution of CDCl3 containing 5% ethyl crotonate is used as a line shape standard by Anasazi Instruments.
Spectra of Ethyl Crotonate Sample
Figure 1 shows the proton spectrum CDCl3 containing 5% dissolved ethyl crotonate, and shows the ultimate resolution capability of the EFT 60/90 spectrometers. The 1H-1H proton coupling is observed in each of the peaks shown in the figure, while residual CHCl3 in the solvent is also seen in the proximity of the high frequency doublet of quartets.
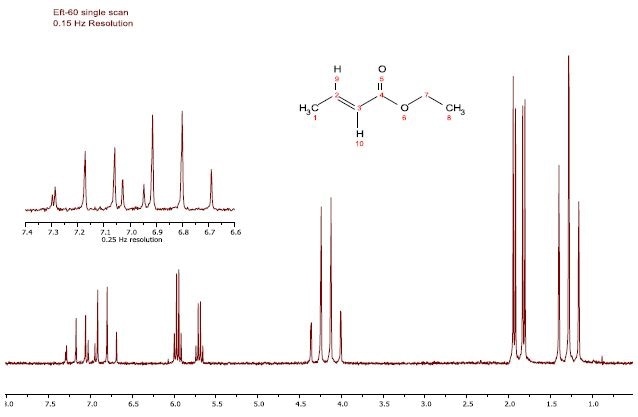
Figure 1. Proton spectrum of 5% Ethyl crotonate in CDCl3.
The 13C and DEPT spectra were obtained using BAPR techniquefor the 5% ethyl crotonate sample, shown in Figure 2. This allows extended data collection on diluted samples without needing to use a field frequency lock. The six resonances and the TMS are seen to be easily resolved by the 13C spectrum, shown in maroon, while the CDCl3 solvent triplet can also be observed.
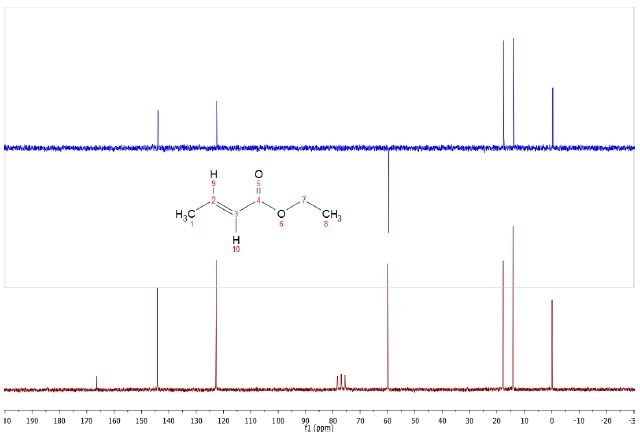
Figure 2. DEPT (top) and C13 (bottom) spectra of 5% Ethyl crotonate in CDCl3.
Carbon atoms with directly attached protons are significantly larger than those without bonded protons (C4 carbonyl). Using 30°+ widths in place of 90° and extending the relaxation delay by 1-2 seconds in can improve these. Due to this, the carbonyl can be seen using standard acquisition parameters.
The single CH2, two methynes and two methyl peaks can be clearly seen in the DEPT-135 spectrum. As would be expected, the peaks due to the solvent and the carbonyl carbon are quenched due to the absence of directly attached protons.
Heteronuclear Correlation
In contrast to the COSY experiment, in the Heteronuclear Correlation experiment (HETCOR) coupling between two different nuclei types is considered. Proton resonances are correlated with those of directly attached carbon-13 or other nuclei in the HETCOR experiment, with the proton represented by the axes of the contour plot.
The chemical shift ranges and signals from other X-nuclei occur at coordinates relative to the shifts of the bonded pairs of nuclei. Rather than detecting the signals from protons, this experiment detects X-nucleus signals. Modifying the delays in HETCOR allows long range coupling information (i.e. couplings between X-nucleus and a proton through 2-3 bonds) to be obtained. The HETCOR spectrum for the 5% ethyl crotonate in CDCl3 is shown in Figure 3.
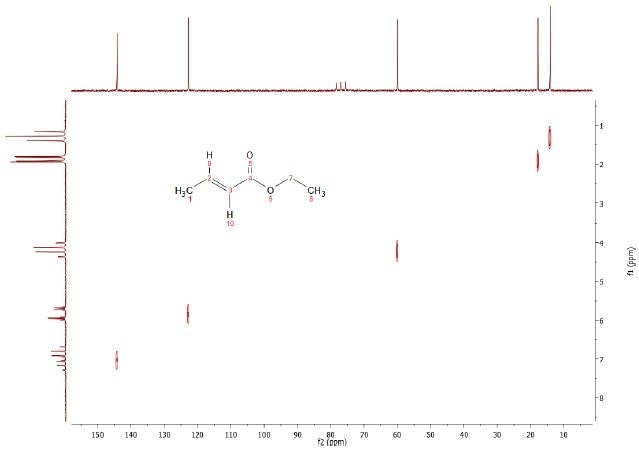
Figure 3. C13-H1 Heteronuclear Correlation (HETCOR) of 5% Ethyl crotonate in CDCl3.
About Anasazi Instruments
 Anasazi Instruments has been providing high quality, rugged, easy-to-use 60 and 90 MHz NMR spectrometers and upgrades to the educational and industrial markets. These instruments have been successfully implemented at hundreds or institutions ranging from large companies and top-tier universities to community colleges throughout North and South America. In research environments, the Eft is a cost-effective workhorse for synthetic and analytical laboratories.
Anasazi Instruments has been providing high quality, rugged, easy-to-use 60 and 90 MHz NMR spectrometers and upgrades to the educational and industrial markets. These instruments have been successfully implemented at hundreds or institutions ranging from large companies and top-tier universities to community colleges throughout North and South America. In research environments, the Eft is a cost-effective workhorse for synthetic and analytical laboratories.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.