Acquired from the human immunodeficiency virus (HIV), lentiviral vectors are one of the most practical and commonly used viral vectors. They provide the ability to transduce both non-dividing and dividing cells and offer a large genetic capacity.
The lentivirus most importantly has the distinct capability to translocate throughout an intact nuclear envelope. There has been significant lentiviral research over the past two decades, and lentiviral vectors have been enhanced to transfer genes into both T cells and stem cells.
Lentiviral vectors offer an encouraging therapeutic choice for a number of conditions, for example, cancers and primary immunodeficiencies.
They also form the foundation of successful anti-viral vaccines and have the possibility to widen the scope of disease protection through vaccination against pathogens that are cell-bound, such as those that are responsible for malaria and tuberculosis.
The manufacture of viral vectors can be difficult and organizations with experience in the necessary methodologies offer vector manufacture services. One such organization is Jinwei, a biotechnology business established in Shanghai.
Jinwei is dedicated to investing in cutting-edge technology to develop and identify cures for patients with life-threatening and severe diseases.
Since it was founded in 2016, the organization has expanded to provide lentiviral vector manufacturing, analytical testing and development for pre-clinical and clinical purposes.
The services offered by Jinwei include the complete lifecycle of lentiviral product development from research, process and analytical methodology refinement to production at full-scale in line with Good Manufacturing Practices (GMP), quality control, fill and finish and regulatory support.
Jinwei’s experience in the manufacture of lentiviral vectors is proven through the development of the DNA flap constructs to optimize the efficacy of transduction.
Jinwei is the only business in China that is licensed to commercially manufacture any gene transfer vector, including the DNA flap sequence.
Jinwei is working in collaboration with the Huashan Hospital to produce innovative, tailored therapies for a range of cancers, including prostate and liver cancers, that target certain genetic aberrations found in a specific tumor.
The organization is also creating anti-viral vaccines and vaccines for different infectious diseases, such as tuberculosis and hepatitis B, where only prophylactic vaccines are available.
Jinwei technology
Jinwei is building on over 20 years of development and research from the Pasteur Institute and Theravectys. This long-term expertise allows Jinwei to supply enhanced vector technology, including exclusive envelopes and a non-integrative backbone that facilitate prime and boost regimens.
The organization has also developed novel technologies that differentiate them from other companies’ services.
For example, incorporating the DNA flap in lentiviral vectors has been proven to enhance the efficiency of transduction by at least 40% in contrast with lentiviral vectors that do not have this sequence, which strongly enhances the efficacy of gene transfer.
Jinwei manufacturing process
Jinwei operates in a modern laboratory and GMP-compliant 1700 m2 manufacturing plant.
The plant adheres to the standards of the key regulation organizations, including the Chinese National Medical Products Administration (NMPA), the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).
The production facility has exclusive single-use semi-automated fill-and-finish equipment and quality control testing features included.
Theravectys received cGMP (Current GMP) status in 2015 by the French National Agency for Drug and Health Product Safety (ANSM). After strategic modifications, Jinwei moved all the platforms of production, including technical documents and production tools, to the new facility in Shanghai.
Jinwei’s extensive cGMP gene therapy services involve the production of lentiviral vectors for both ex-vivo and in-vivo applications.
All material is created in line with the requirements of the main regulatory organizations.
The manufacturing procedure involves purification stages and a sterilizing filtration stage prior to filling (Figure 1). The manufacturing method is compatible with direct human injections.
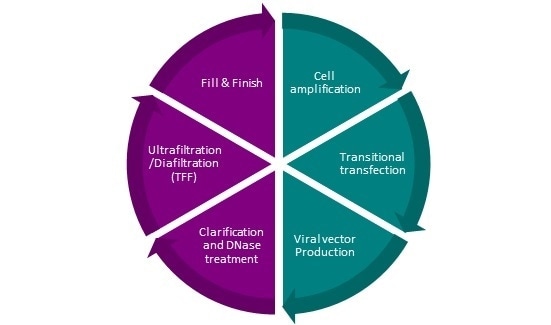
Figure 1. Jinwei lentiviral vector manufacturing process. Image Credit: Jinwei Bio
The facility in Shanghai’s Zhangjiang Cell Therapy Park is the location for all of Jinwei’s lentiviral vector manufacture, which has a finishing capacity of up to 5000 vials. It is based on a fully disposable process line technology and is operated by a globally experienced GMP team.
Along with this, a greater amount of flexibility is provided by independent production,
To conclude, Jinwei has developed procedures that allow the supply of efficient, dependable and safe viral vector-based products, which in turn may assist in translating early-stage studies into clinically viable therapies.
References
- Jinwei. http://www.jinweibio.com
- Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol Ther Methods Clin Dev. 2016.
About Jinwei Bio
 Shanghai Jinwei Biotechnology Co., Ltd. (Jinwei) is a biotechnology company dedicated to pursuing innovative technology to develop cures for people with serious and life-threatening diseases.
Shanghai Jinwei Biotechnology Co., Ltd. (Jinwei) is a biotechnology company dedicated to pursuing innovative technology to develop cures for people with serious and life-threatening diseases.
Jinwei has obtained exclusive licenses of multiple patents and other intellectual property owned by the Pasteur Institute and Theravectys for development, manufacture and commercialization of therapeutic products based on lentiviral vectors.
We offer lentiviral vector development, manufacturing and analytical testing for clinical and commercial supply. Our services include Process and Analytical Methods Development, Full GMP Production, with USP and DSP, Fill & Finish, Quality Control & Quality Assurance and Regulatory Support.
The GMP production facility located in Shanghai is dedicated to provide safe and reliable viral vector-based products and CMO/CDMO services, helping to translate early stage research into commercially viable therapies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.