Sponsored Content by GenScriptReviewed by Maria OsipovaNov 16 2022
Chinese Hamster Ovary cells also referred to as “CHO cells,” have a long history of being used as research tools. Since their introduction to biomedical research in the 1950s, these cells have become the most popular non-human mammalian cell line for the production of biological therapies (i.e., monoclonal antibodies, enzymes, cytokines, and hormones).
For instance, CHO cells were used to create the vast majority (~84%) of monoclonal antibody therapies licensed between 2014 and 2018. Indications for the use of monoclonal antibodies produced from CHO cells include the treatment of multiple sclerosis, HIV, asthma, cancer, and neuroblastoma.
Other mammalian cell lines commonly utilized in bioprocessing include NS0 or Sp2/0 murine myeloma cells and baby hamster kidney (BHK21) cells (Chin et al. 2019). The resilience to growth conditions, viral infection resistance, and high protein synthesis capability of CHO cells, however, have all contributed to their growing application in bioprocessing.
Significantly, the post-translational modifications (such as glycosylation) and folding of human proteins are more closely conserved when proteins are processed by CHO cells.
A more recent development in bioprocessing is the increased usage of human cell lines, notably the human embryonic kidney 293 (HEK293) cells and the human sarcoma cell line HT-1080, among many others (Chin et al. 2019).
This strategy is becoming more and more popular since non-human mammalian cell lines cannot completely mimic human-like glycosylation patterns. For instance, GnT-III, Gal alpha2,6 ST, and alpha 1,3/4 fucosyltransferase, which are present in human cells, are not expressed in CHO cells (Goh and Ng, 2018).
Additionally, mammalian cell lines like CHO cells create post-translational changes, such as the insertion of alpha-gal and NGNA glycans, that are absent from human proteins. In the end, these non-human glycosylations raise the possibility of immunological responses to biotherapeutics (Dumont et al. 2016).
Deficits in human-like glycosylation patterns can also have a detrimental impact on manufacturing processes and treatment efficacy since post-translational alterations affect the yield, activity, and pharmacokinetic characteristics of recombinant proteins.
HEK293 vs. CHO cell lines in bioprocessing
One of the most prominent human cell lines for the synthesis of therapeutic proteins is HEK293-derived clones. HEK293 cell clones have been identified or produced by genetic engineering and offer better characteristics, such as higher protein output, transfection efficiency, and growth rate.
The increased potential of virus contamination and subsequent transmission when utilizing human cell lines is possibly the most important constraint.
There have been many CHO cell lines created to date; the CHO-K1 and CHOK1SV cell lines are widely employed to create biotherapeutics. Genetically modified CHO cell lines have improved glycosylation, decreased apoptosis, and increased productivity (Zhu et al. 2017).
While there are certain drawbacks to employing non-human mammalian cells in bioprocessing, there is enough proof of the safety of CHO cells to last decades. Glycoproteins that are active and well-tolerated by patients have been successfully produced using CHO cells (Jayapal et al. 2007).
As a result, it is anticipated that CHO cells will continue to dominate the bioprocessing workflow, supported by a clear path toward regulatory clearances.
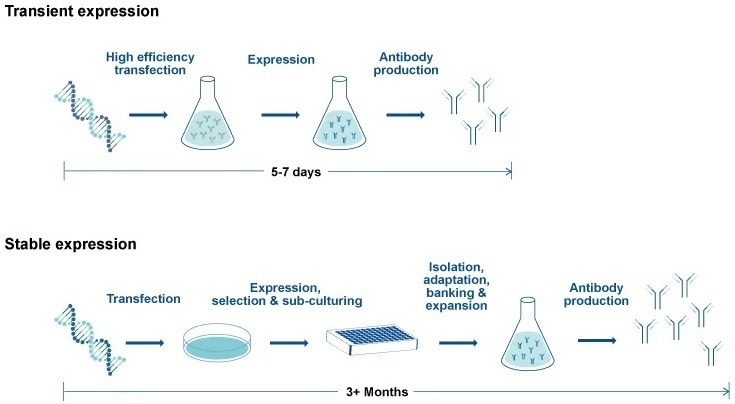
Comparison of workflow and timeline for transient vs. stable antibody expression. Image Credit: GenScript
From transient to stable CHO expression
Ultimately, cell line selection for bioprocessing is impacted by factors such as the protein-type being produced and the recombinant protein's glycosylation profile. For proteins generated by CHO vs. HEK293 cells, significant variations in glycosylation and glycostructure have been found (Croset et al. 2012, Goh and Ng, 2018).
As a result, it is important to employ a specific cell expression method consistently throughout the early and late phases of the production of biotherapeutic proteins.
For instance, the use of CHO transient expression speeds up the production of smaller quantities of antibody candidates, which are perfect for initial in vitro testing in the creation of therapeutic monoclonal antibodies.
For subsequent preclinical phases, higher-yield stable CHO expression of lead recombinant monoclonal antibodies is advised. This method increases the liklihood that candidates for pharmacological and toxicological characterization conserve established glycosylation patterns supporting IND and therapeutic development (Jain et al. 2017, Sifiniotis et al. 2019).
References
- Chin, C. L. et al. A human expression system based on HEK293 for the stable production of recombinant erythropoietin. Sci. Rep. (2019) doi:10.1038/s41598-019-53391-z
- Croset, A. et al. Differences in the glycosylation of recombinant proteins expressed in HEK and CHO cells. J. Biotechnol. (2012) doi:10.1016/j.jbiotec.2012.06.038
- Dumont, J., Euwart, D., Mei, B., Estes, S. & Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Critical Reviews in Biotechnology (2016) doi:10.3109/07388551.2015.1084266
- Goh, J. B. & Ng, S. K. Impact of host cell line choice on glycan profile. Critical Reviews in Biotechnology (2018) doi:10.1080/07388551.2017.1416577
- Jain, N. K. et al. A high density CHO-S transient transfection system: Comparison of ExpiCHO and Expi293. Protein Expr. Purif. (2017) doi:10.1016/j.pep.2017.03.018
- Jayapal, K. P., Wlaschin, K. F., Hu, W. S. & Yap, M. G. S. Recombinant protein therapeutics from CHO Cells - 20 years and counting. Chem. Eng. Prog. (2007)
- Sifniotis, V., Cruz, E., Eroglu, B. & Kayser, V. Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies (2019) doi:10.3390/antib8020036
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. (2018) doi:10.1038/nbt.4305
- Zhu, M. M., Mollet, M., Hubert, R. S., Kyung, Y. S. & Zhang, G. G. Industrial Production of Therapeutic Proteins: Cell Lines, Cell Culture, and Purification. in Handbook of Industrial Chemistry and Biotechnology (2017). doi:10.1007/978-3-319-52287-6_29
About GenScript
Genscript is the world’s leading biotech company providing life sciences services and products. With gene synthesis, peptide, protein, antibody and preclinical drug development service capabilities, we are internationally recognized as a leading biotech company specializing in fundamental life sciences research and early-phase drug discovery services. As of 2018, more than 30,000 peer-reviewed journal articles cited GenScript’s services and products, making GenScript the most frequently cited biotech company in the world.
After almost two decades of fast growth in developing biological reagents, the company has expanded its business into immunotherapy, CDMO, laboratory equipment, and microbial industry to further fulfill its mission in making people and nature healthier through biotechnology.
Founded in 2002 in New Jersey, United States, GenScript serves as a partner for researchers in basic life sciences, translational and biomedical fields as well as early-stage drug development.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.