Sponsored Content by GenScriptReviewed by Maria OsipovaNov 16 2022
Non-alcoholic fatty liver disease (NAFLD) is becoming increasingly associated with Metabolic Syndrome, a collection of metabolic defects that promote cardiovascular disease. NAFLD encompasses a variety of chronic liver disease states, beginning with steatosis and advancing to cirrhosis, which entails persistent liver tissue damage (Engelmann and Tacke, 2022).
NAFLD is predicted to have a global prevalence of more than 30%, with an average of ~47 new cases per 1000 people on an annual basis. Statistically, men have a higher yearly incidence of NAFLD (~70/1000) than women (30/1000) (Riazi et al. 2022).
Worldwide, NAFLD is the most frequent kind of chronic liver disease. Despite the fact that several clinical trials are now being conducted to evaluate various pharmacological options targeting pathogenic processes, no medicines have yet been authorized by the FDA for NAFLD.
As a result, the treatment of NAFLD and nonalcoholic steatohepatitis (NASH) patients are reliant on diet, lifestyle changes, exercise, and medicines recommended for obesity, diabetes, inflammation, and oxidative stress (Negi et al. 2021).
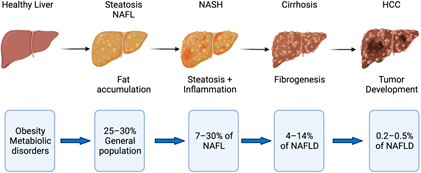
Disease progression in fatty liver disease. Obesity and metabolic disorders represent risk factors for NAFLD, which starts with fat accumulation in hepatocytes. Further disease progression is characterized by inflammation (NASH) and subsequent fibrogenesis leading to cirrhosis. NASH with and without cirrhosis bears the risk of developing hepatocellular carcinoma (HCC). Image Credit: Retrieved without modifications from Engelmann and Tacke, 2022.
Pathogenic mechanisms that underscore NAFLD and its progression to NASH
NAFLD pathogenesis has long been thought to result from excessive lipid buildup within hepatocytes, known as steatosis, which is a defining feature of the illness. Hepatic steatosis is caused by an imbalance in the influx and use of fatty acids by the liver.
Disproportionate fatty acid buildup in the liver results from three major sources: lipolysis, lipogenesis, and diet. Once metabolic abnormalities are evident, further stressors, such as lipotoxicity and inflammation causing liver injury, hasten the transition from NAFLD to NASH (Negi et al. 2021).
Biotherapeutics under clinical studies for NAFLD
Numerous investigational drugs, including small molecules and biotherapeutics, are being clinically tested against important targets involved in the pathophysiology of NAFLD-NASH. Protein analogs (like FGF19 and FGF21), bispecific antibodies (like FGFR1/KLB), and peptide receptor agonists (like GLP-1/glucagon) are a few of the biotherapeutics being tested.
Collectively, FGF19 and FGF21-based drugs have been found to lower body weight and enhance lipid metabolism in preclinical and clinical investigations (Talukdar and Kharitonenkov 2021).
The AAV gene delivery approach that targets lipid oxidation
Researchers at the University of Virginia School of Medicine used an AVV8 vector to trigger the expression of a single-chain variable fragment of E06 (E06-scFv) in hepatocytes of a diet-induced NAFLD/NASH mouse model (Upchurch et al. 2022).
As a result of the excess radical oxygen species generated in the liver under steatosis conditions, increased lipid oxidation products such as oxidized phosphatidylcholines (OxPCs) form part of the pathology of NAFLD/NASH.
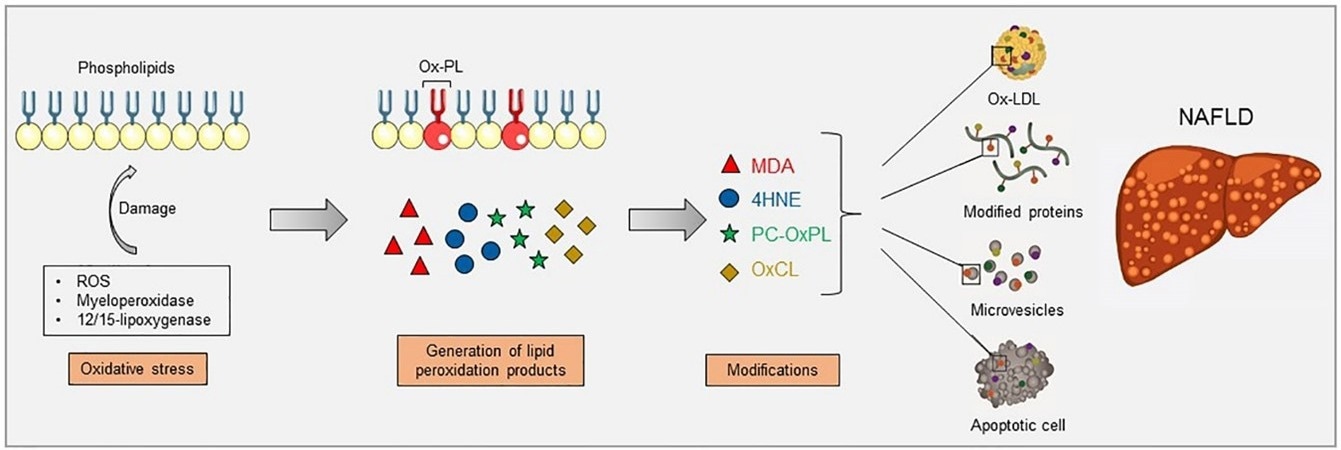
Increased oxidative stress causes lipid peroxidation, which can occur via enzymatic reactions, such as myeloperoxidase and 12/15-lipoxygenase, and non-enzymatic reactions, such as reactive oxygen species (ROS). Lipid peroxidation of membrane phospholipids results in their fragmentation and the generation of breakdown products which can further modify free amino groups of proteins and lipids, forming covalent adducts and creating oxidation-specific epitopes (OSEs), including malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), phosphocholine on oxidized phospholipids (PC-OxPL), and oxidized cardiolipin (OxCL). These epitopes are carried by oxidized low-density lipoproteins (OxLDL), modified proteins, microvesicles, and apoptotic cells, aspects that have been shown to be present during NAFLD. Image Credit: Retrieved without modifications from Figure 1 Hendrikx and Binder 2020.
E06 is an IgM that occurs naturally and targets and nullifies oxidized phosphatidylcholines (OxPCs). Joseph Witztum’s team at the University of California, San Diego, had previously demonstrated proof of principle for utilizing E06-scFv to target OxPCs.
The Witztum group revealed in their research that transgenic expression of E06-scFv in a mouse model of NASH successfully lowered steatosis, inflammation, fibrosis, cell death, and mitochondrial dysfunction, as well as lowered lipid accumulation in hepatocytes (Sun et al. 2019).
As a result, Upchurch et al. used a similar technique, but instead of constitutively expressing E06-scFv, the scientists used an AAV8 vector to promote hepatic expression of the antibody fragment.
We developed a cre-dependent adeno-associated viral construct for expression of scFv-E06. The E06 coding region was synthesized by GenScript from the publicly available, published sequence (Piscataway, NJ, USA), containing a C-terminal myc- and His-tag and flanking 5′ Mlu I and 3′ Nhe I restriction sites.
Upchurch et al. 2022
Ultimately, researchers were able to demonstrate that early AAV8-scFv-E06 expression can prevent lipid accumulation. AAV8-scFv-E06 expression at a later disease stage, on the other hand, helps decrease fibrosis and progression to NASH after steatosis is established.
Furthermore, the scientists linked these favorable results to a decrease in certain OxPC species, offering researchers an opportunity to learn more about the molecular processes that may have an impact on the onset of NAFLD and its progression to NASH.
References
- Engelmann, C. & Tacke, F. The Potential Role of Cellular Senescence in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. (2022) doi:10.3390/ijms23020652.
- Hendrikx, T. & Binder, C. J. Oxidation-Specific Epitopes in Non-Alcoholic Fatty Liver Disease. Frontiers in Endocrinology (2020) doi:10.3389/fendo.2020.607011.
- Negi, C. K., Babica, P., Bajard, L., Bienertova-Vasku, J. & Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism: Clinical and Experimental (2022) doi:10.1016/j.metabol.2021.154925.
- Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. The Lancet Gastroenterology and Hepatology (2022) doi.org/10.1016/S2468-1253(22)00165-0.
- Sun, X. et al. Neutralization of Oxidized Phospholipids Ameliorates Non-alcoholic Steatohepatitis. Cell Metab. (2020) doi:10.1016/j.cmet.2019.10.014.
- Talukdar, S. & Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Molecular Metabolism (2021) doi:10.1016/j.molmet.2020.101152.
- Upchurch, C. M., et al. Targeting oxidized phospholipids by AAV-based gene therapy in mice with established hepatic steatosis prevents progression to fibrosis. Science DOI: 10.1126/sciadv.abn0050.
About GenScript
Genscript is the world’s leading biotech company providing life sciences services and products. With gene synthesis, peptide, protein, antibody and preclinical drug development service capabilities, we are internationally recognized as a leading biotech company specializing in fundamental life sciences research and early-phase drug discovery services. As of 2018, more than 30,000 peer-reviewed journal articles cited GenScript’s services and products, making GenScript the most frequently cited biotech company in the world.
After almost two decades of fast growth in developing biological reagents, the company has expanded its business into immunotherapy, CDMO, laboratory equipment, and microbial industry to further fulfill its mission in making people and nature healthier through biotechnology.
Founded in 2002 in New Jersey, United States, GenScript serves as a partner for researchers in basic life sciences, translational and biomedical fields as well as early-stage drug development.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.