AAV production requires highly accurate, precise, and high-throughput determination of AAV titer and empty vs. full ratio (E/F ratio) to ensure efficacy, safety and quality.
Gator Bio’s Next Gen biolayer interferometry (BLI)-based AAV solutions determine AAV capsid titer and empty versus full content without needing to rely on additional techniques (e.g., ELISA, qPCR, ddPCR, AUC, and TEM) to obtain data on different AAV critical quality attributes (CQA).
This article will present data from the Gator AAV analytics for Titer and E/F Ratio. Gator Bio’s solutions generate reproducible, accurate data suitable for upstream and downstream samples.
Features of Gator’s AAV analytics solutions
- Kinetic characterization of AAV antibodies
- Automated and robust titer in the range of 1x107 to 1x1013 vp/mL
- High throughput, automated empty versus full ratio determination
High sensitivity “Dip and Dilute” titer of upstream samples
The HS AAV kit enables:
- Titer of AAV samples down to 1x107 vp/mL for all AAV serotypes
- Improved sensitivity thanks to patented signal amplification technology
- The platform provides accuracy, automation, and high precision
Image Credit: Gator Bio, Inc.
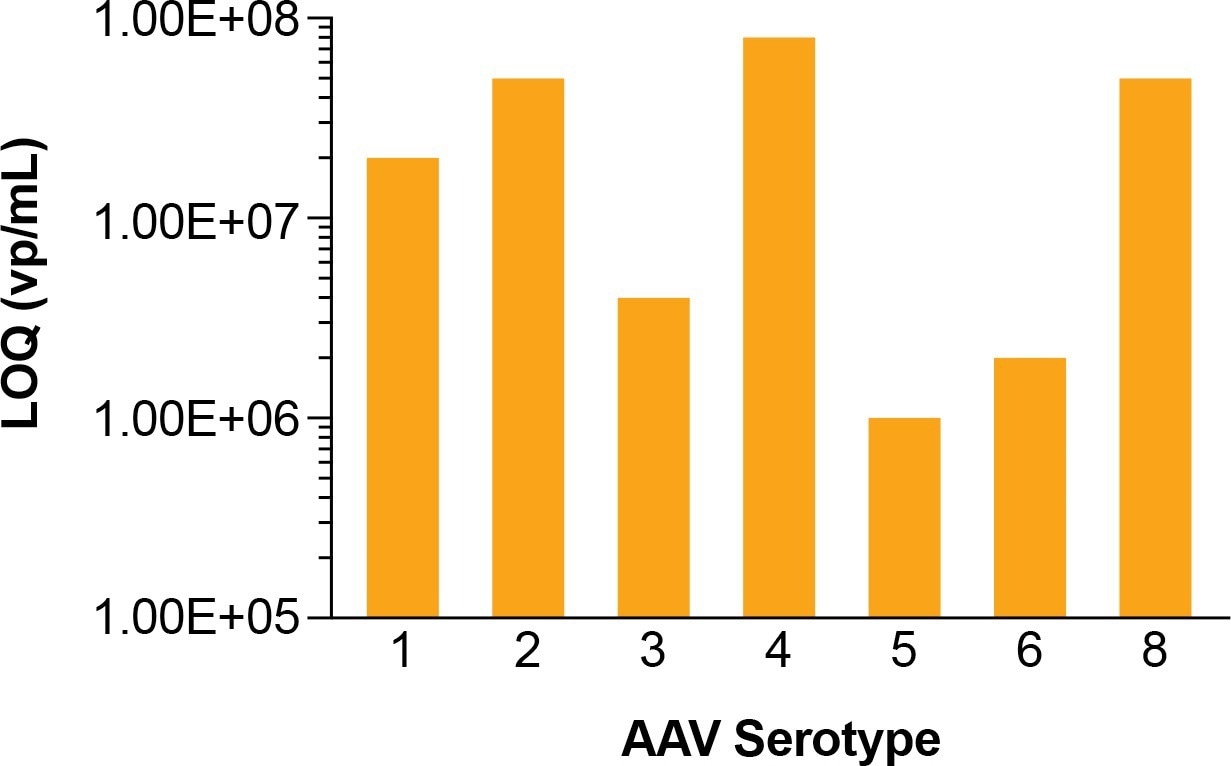
Figure 1. LOQ for AAV1-8. Serotypes are purchased from www.virovek.com. Image Credit: Gator Bio, Inc.
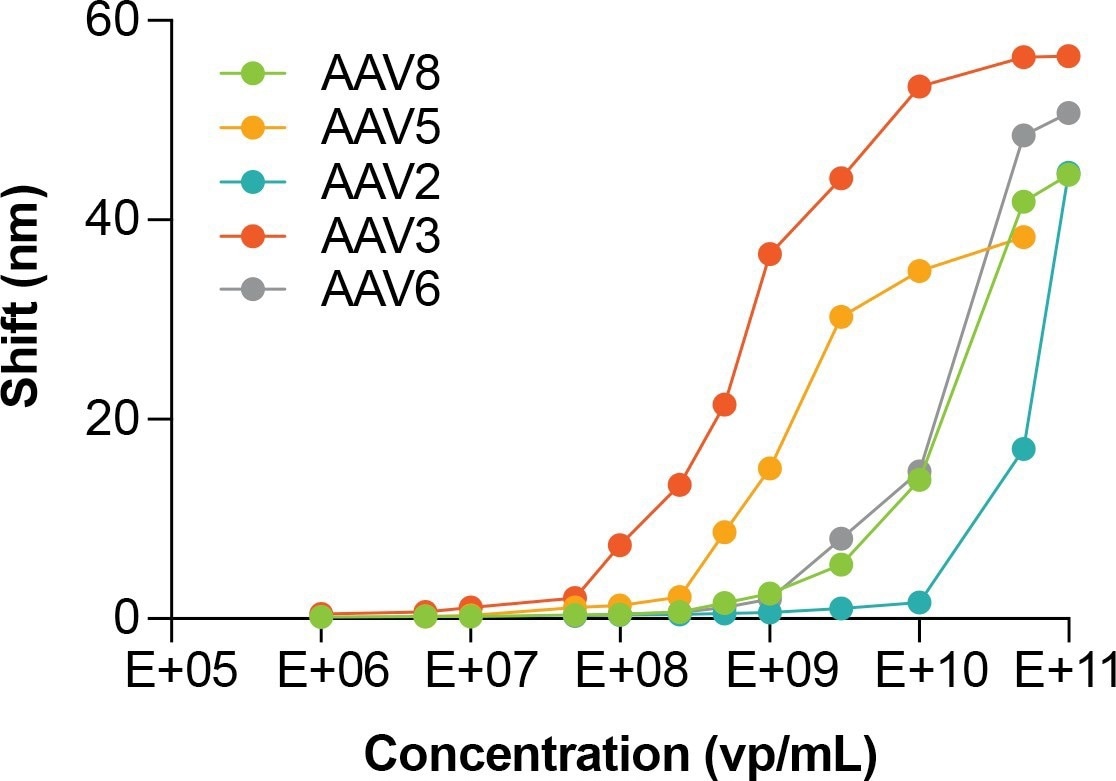
Figure 2. HS AAV dynamic range for different AAV serotypes. Image Credit: Gator Bio, Inc.
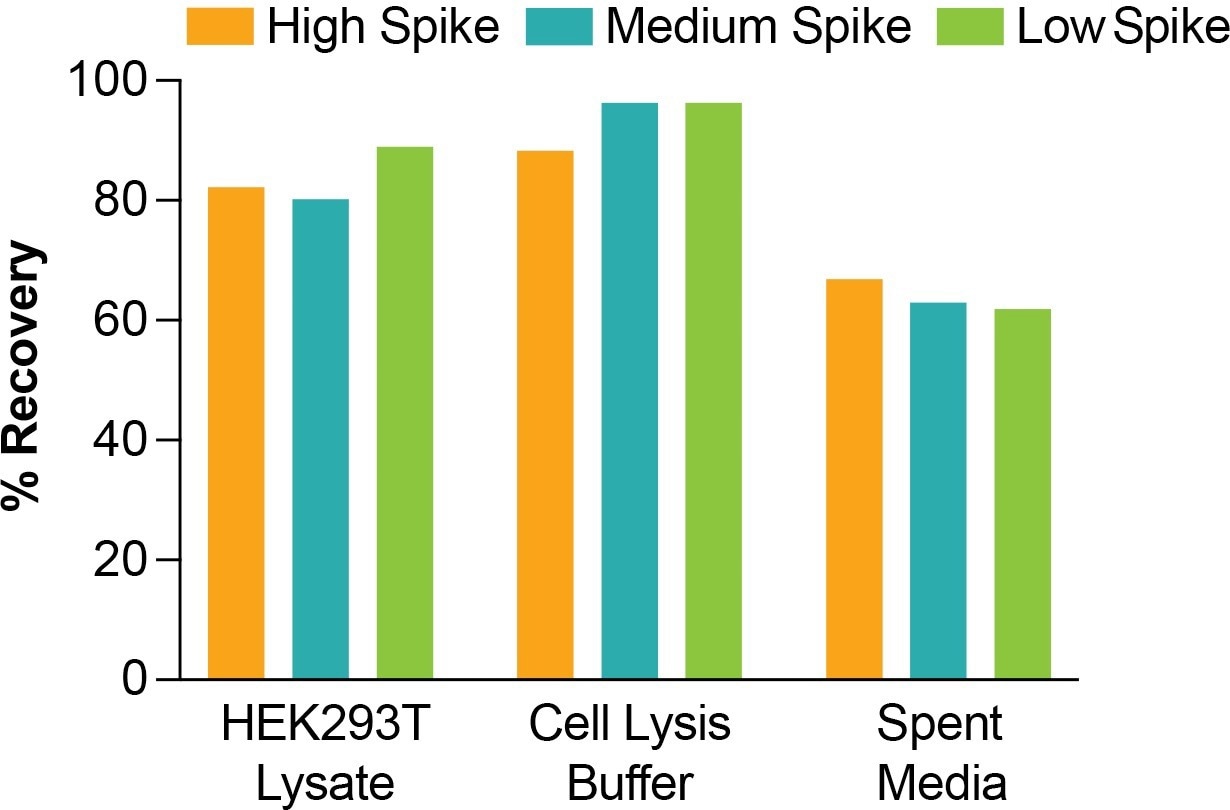
Figure 3. Recovery of AAV spiked at high (1x108 vp/mL), medium (5.33107 vp/mL), and low (1x107 vp/mL) concentrations in various sample matrices. Image Credit: Gator Bio, Inc.
Table 1. Source: Gator Bio, Inc.
Direct binding titer of downstream samples
- Titer is based on the binding rate of the AAV capsid of interest to the AAVX probe surface.
- The probes can be reused at least 10 times with regeneration without compromising performance.
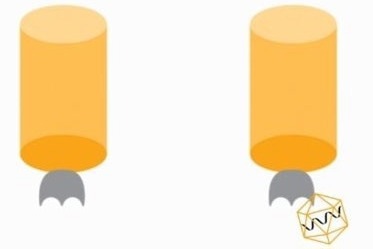
Image Credit: Gator Bio, Inc.
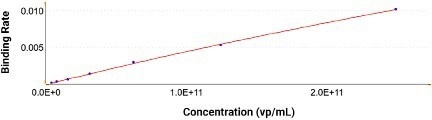
Figure 4. Standard curve for AAV2 generated using AAVX probe. Image Credit: Gator Bio, Inc.
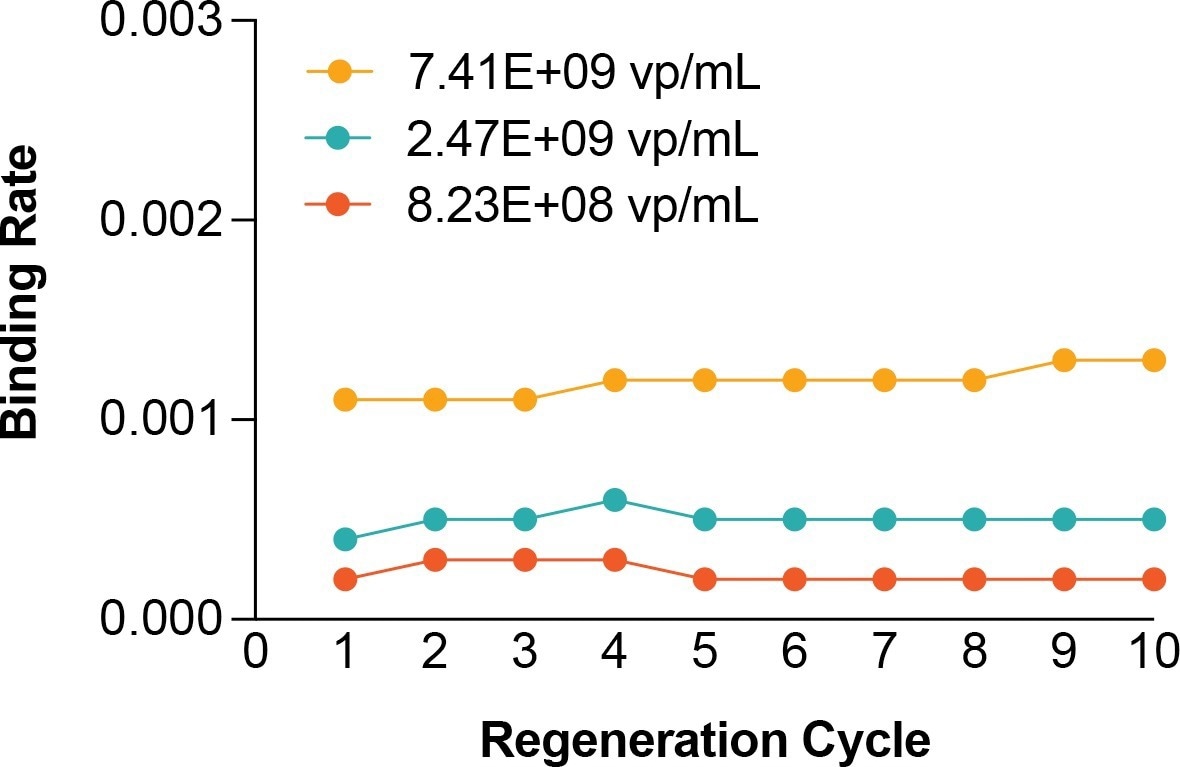
Figure 5. There is no loss in performance after 10 regenerations using the same AAVX probe. Image Credit: Gator Bio, Inc.
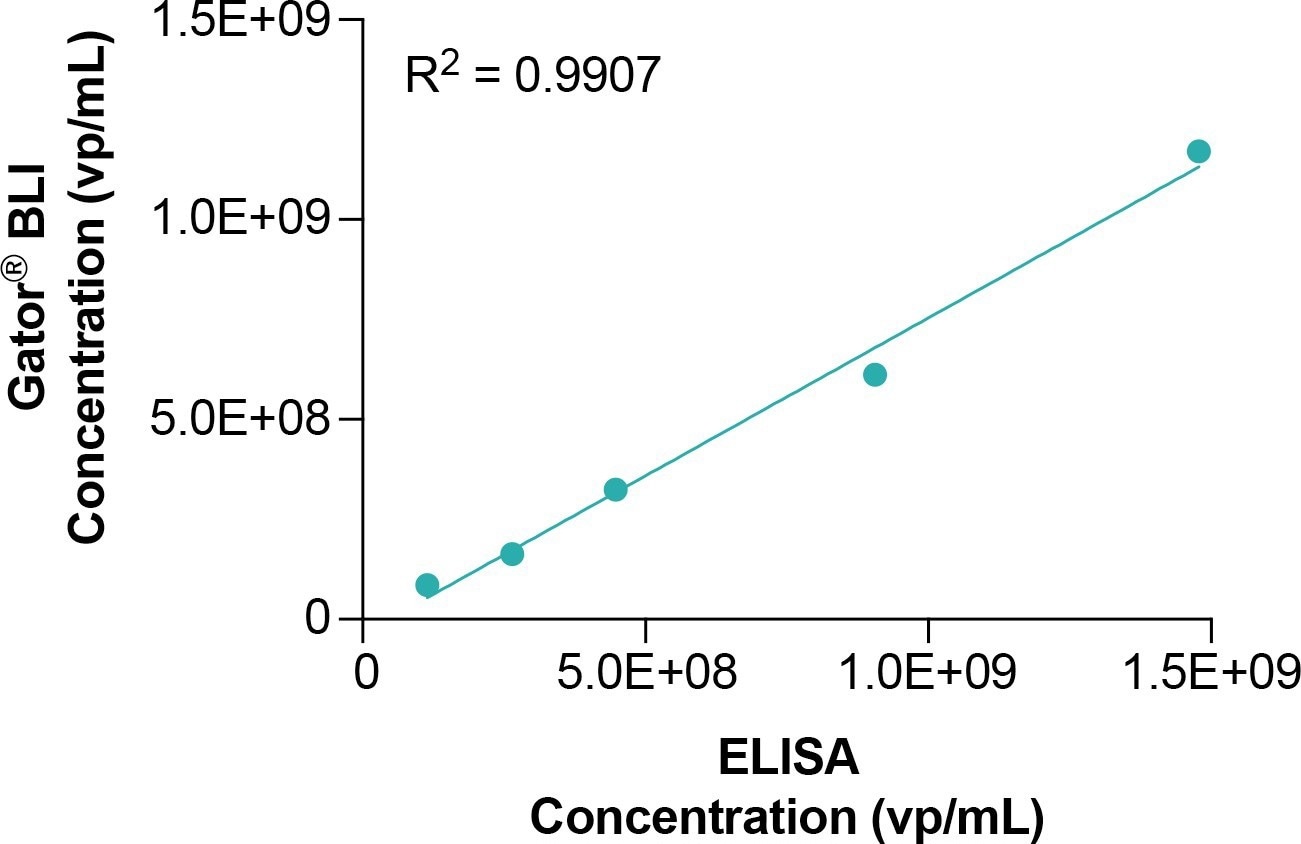
Figure 6. Correlation between the quantification of AAV9 obtained with AAVX probes versus ELISA. Image Credit: Gator Bio, Inc.
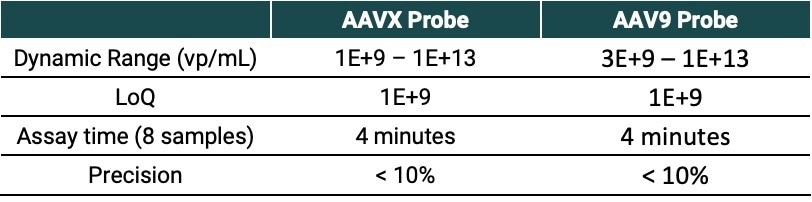
Table 2. Source: Gator Bio, Inc.
Empty/full AAV ratio determination
Aside from AAV titer measurement, assays are suitable for E/F ratio determination.
The assay has three steps:
- AAV capture using AAVX probes
- Lysis to release ssDNA and
- Capture of ssDNA on ssDNA probes. Quantitation of ssDNA released from AAV capsids determines the % full ratio measurement.
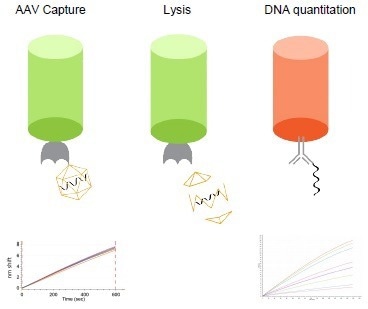
Image Credit: Gator Bio, Inc.
A single assay is used for E/F ratio determination, meaning that the kit is easy to use. No other methods are necessary, making it a highly efficient, inexpensive, and reliable standalone system.
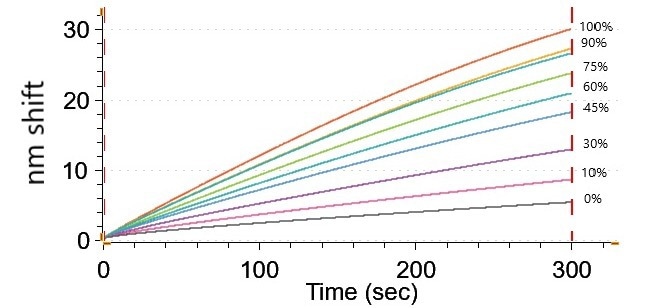
Figure 7. Sensogram showing ss DNA binding to DNA binding probe. The nm shift is proportional to the amount of DNA. Image Credit: Gator Bio, Inc.
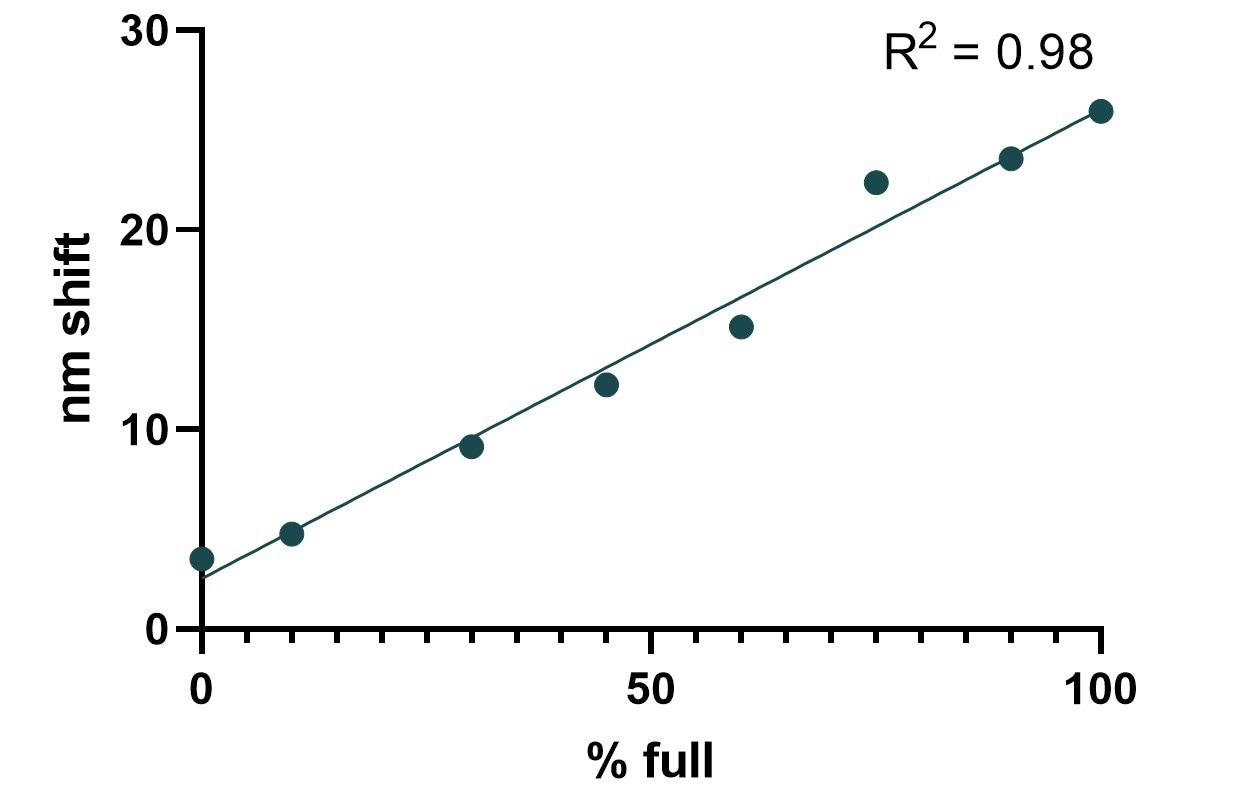
Figure 8. Standard curve for % full capsids in an AAV2 sample. Image Credit: Gator Bio, Inc.
Table 3. Source: Gator Bio, Inc.
Determining total AAV antibodies in serum
Gator Bio’s equipment provides a simple and automated workflow for the determination of total AAV antibodies in a serum sample. A signal amplification format is used in AAVX probes.
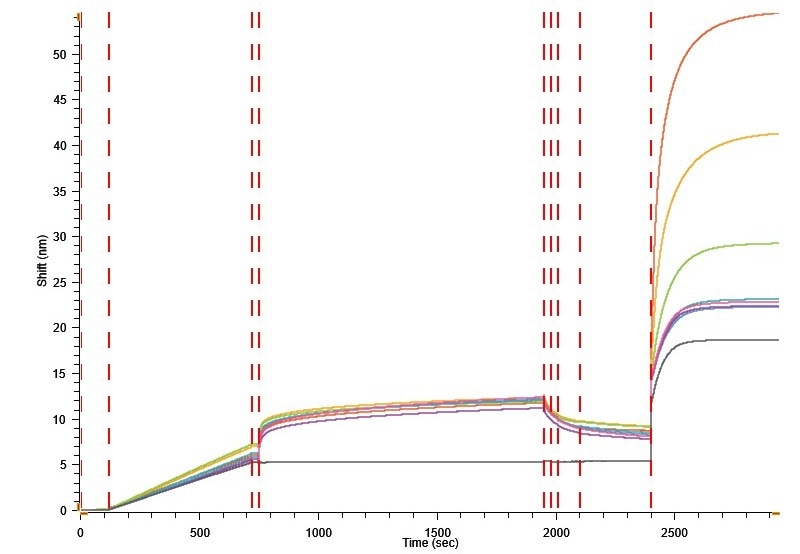
Figure 9. Human serum(1:50) diluted in Q spiked ADK9 antibody. Image Credit: Gator Bio, Inc.
Gator® AAV Analytics Solution Guide
Table 4. Source: Gator Bio, Inc.
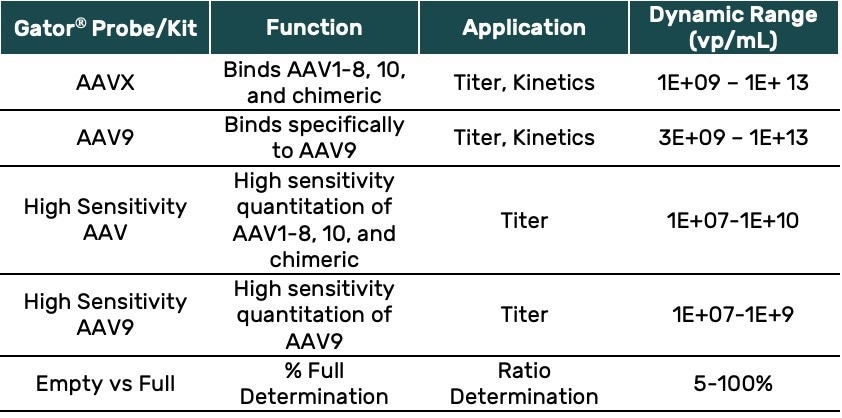
Advantages of Gator® AAV Analytics
- Wide dynamic range of 1x107 to 1x1013 vp/mL
- Matrix compatibility ideal for upstream analysis
- Single assay for empty/full AAV ratio determination
- Kinetics characterization of AAV neutralizing antibodies
- Qualitative and quantitative determination of antibodies to AAV serotypes in plasma and serum
- Automation
- Suitable for integration into manufacturing processes
- Cost savings from a single technology for AAV titer and content ratio determination
Summary
Gator® AAV probes and kits combined with BLI technology provide a total AAV analytics solution for gene therapy products and both upstream and downstream samples.
About Gator Bio, Inc.
Gator Bio is a world-leading biosensor company headquartered in Palo Alto, CA. At Gator Bio, we provide researchers the tools and instrumentation to advance their research. From antibody engineering to small molecule drug discovery to basic research, Gator Bio can be used to bring meaning to the unknown. From the original inventors of label-free biolayer interferometry (BLI), Gator Bio provides the next generation of BLI technology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.