Sponsored Content by Bio-TechneReviewed by Olivia FrostJan 8 2024
Bio-Techne’s analytical platforms provide the automation and scalability required for the development and manufacture of cell and gene therapy vectors. Low volume requirements enable the preservation of valuable samples. Platform methods can be effortlessly transferred across laboratories and project stages (from discovery to manufacturing), providing consistent characterization results from start to finish.
Simple Western™: Multi-attribute analysis
- Multi-attribute Adeno-associated virus (AVV) characterization
- Quantify empty/full content ratio
- Lentiviral analysis made simple

Image Credit: Bio-Techne
Simple Plex™: Your next-generation ELISA
- Fast, sensitive, and reliable AAV capsid titration
- HIV-1 Gag p24 cartridge
- HEK 293 host cell protein cartridge

Image Credit: Bio-Techne
Maurice™: One-stop cIEF and CE-SDS
- Stability, identity, and purity all in one
- See what is inside your AVV capsids
- Easily measure surface charge for formulation development

Image Credit: Bio-Techne
Maurice™: One-stop cIEF and CE-SDS
- Identify AAV aggregates
- Detect AAV contaminants
- Monitor ultrafiltration on AAV preparation

Image Credit: Bio-Techne
Adeno-associated virus
There is no need to waste important AAV samples. Bio-Techne’s automated, high-throughput systems allow the characterization of AAVs in sample volumes as low as 3 µL while maintaining high levels of reproducibility and sensitivity. Receive multi-attribute measurements of AAV titer, empty/full content ratio, identity, stability, capsid protein ratio, purity, and more.
Multi-attribute AAV characterization
Utilizing just 3 µL of AAV sample, Simple Western on Jess™ and Abby™ can deliver a wide array of information, such as Critical Quality Attributes (CQAs), which must be monitored during AAV manufacturing.
No sample cleanup is needed. Simple Western can accommodate complex sample types, such as whole-cell lysates, and has the automation and throughput required to scale with AAV manufacturing workflows.
- Identity Immunoassays and Sensitive Total Protein Detection
- Capsid Protein Ratio and Titer Quantification
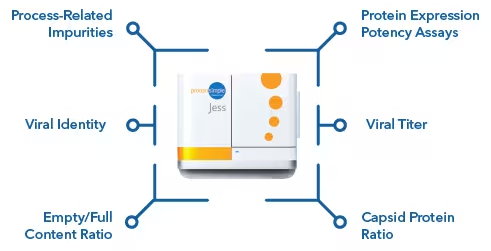
Image Credit: Bio-Techne
Quantify empty/full content ratio
The Simple Western method automatically segregates AAV samples according to their charge or size. This is followed by sensitive and specific detection using anti-DNA and anti-VP1/2/3 antibodies directly in the capillary, enabling the reproducible and quantitative measurement of these central AAV components.
- Do Your AAVs Contain DNA? The Empty/Full Assay
Fast, sensitive, and reliable AVV capsid titration
Introducing the Simple Plex AAV assay, Bio-Techne has partnered with market leader PROGEN to automate the AAV titration ELISA to deliver rapid and efficient viral quantitation with superior specificity. Ella enables accelerated cell and gene therapy workflows.
A PROGEN-ProteinSimple partnership
For those interested in learning more about the recovery, linearity, and dynamic range of the new Simple Plex AAV assay, a downloadable application note that shows how the PROGEN-ProteinSimple partnership surpasses expectations with the novel Simple Plex AAV assay is available below.
- The Simple Plex AAV Viral Titer Assay

Image Credit: Bio-Techne
Purity: HEK 293 HCP 3G Assay
Ella delivers exceptional sensitivity and an expanded dynamic range to ensure precise sample detectability throughout the purification process. The assay is designed around the antibodies used in the Cygnus HEK 293 HCP ELISA kit, 3G Resupply 1 (F650S). Users therefore get the accuracy and consistency of the Cygnus ELISA, but with an automated, streamlined workflow.
- The Simple Plex HEK 293 HCP 3G Cartridge
Image Credit: Bio-Techne
Stability, identity, and purity—All on one system
The purity, stability, and identity of the AAV vector are important aspects of delivering safe gene therapy. The Maurice system combines CE-SDS analysis and imaged capillary isoelectric focusing (icIEF), allowing the compilation of a lot of data into a single, compact system. Up to 100 samples can be loaded per batch. Results are ready in 6-25 minutes utilizing sample volumes as low as 50 µL.
- icIEF Analysis of AAV Proteins for Gene Therapy
- Characterization of AAV Vector Proteins Using Maurice CE-SDS

Image Credit: Bio-Techne
See what is inside your AAV capsids
It can be difficult to determine whether AAV capsids are full, partially full, or empty. This is made simple with Maurice, as it is equipped with icIEF detection. The application note describes how to develop a reproducible method for AAV characterization. It also outlines how this method is stability-indicating and can offer crucial information on the capsid's DNA content (with Maurice’s native fluorescence detection and absorbance modes).
- Assessing AAV Product Quality? Get the Confidence You Need
Identify AAV aggregates and contaminants
Monitoring aggregates of AAV capsids is crucial in securing the stability of the vector. However, methods like microscopy and light obscuration lack throughput and are limited in their ability to detect translucent aggregates.
Bio-Techne’s MFI systems directly image subvisible particles in solution to provide data for ten unique morphological parameters, allowing the identification of AAV aggregates as well as any impurities or contaminants in the sample.
Image Credit: Bio-Techne
Monitor the effect of ultra-filtration on the preparation of AAVs
Voyager Therapeutics’ research team utilized MFI to measure the effect of ultra-filtration on subvisible particle formation. This includes measuring the size and concentration of subvisible particles that formed in solution. This inaugural study provides a quantitative understanding of the ultra-filtration behavior of AAVs for gene therapy to guide process development.
- Ultra-filtration Behavior of Recombinant AAV Vectors
Lentivirus
All-in-one titer, capsid content, stability, purity, and transduction assays
Simple Western offers genuinely multi-attribute analysis for lentivirus vector characterization that scales with manufacturing workflows. Jess is a singular instrument for the quantification of titer, capsid content, stability, purity, and transduction efficiency—even in complex sample types such as whole-cell lysate.
As an open platform, any target of interest can be analyzed with conventional antibodies. Quantify purity utilizing anti-HCP antibodies or total protein detection with sensitivity superior to SDS-PAGE stains (such as SYPRO Ruby). Reduce initial financial outlay and overall costs, including time and labor, to bring novel cell and gene therapies to market.
- An Automated Multi-Attribute Platform for Scalable At-Line Lentiviral Analytics
Image Credit: Bio-Techne
Simple Plex lentivirus titer and purity assays
Titer: p24 Assay
The Simple Plex assay for the identification of human HIV-1 Gag p24 simultaneously utilizes high throughput with superior precision and accuracy to allow for fast quantification of lentiviral capsid proteins during various stages of cell and vector manufacturing.
The Simple Plex p24 assay is automated and reproducible, facilitating the reduction of user error, and therefore making it a perfect tool in good manufacturing practice (GMP) environments, where there is a need for quality, standardization of process, scalability, and assay transfer.
- The Simple Plex HIV-1 Gag p24 Cartridge

Image Credit: Bio-Techne
Image Credit: Bio-Techne
Lipid Nanoparticles (LNP)
Easily measure surface charge for formulations
The safety and efficacy of therapeutics rely on characterizing the identity and stability of LNP delivery vectors. Measuring the surface charge of these large, complex particles is simplified with Maurice. Merck researchers used Maurice during the process and formulation development of an LNP-based mRNA vaccine.
- Characterizing the Surface Charge of LNPs with icIEF
Image Credit: Bio-Techne
Discover how cIEF and CE-SDS on Maurice are utilized in manufacturing to evaluate the purity and stability of AAVs and the identity of LNP in this webinar, featuring John Loughney, Director, Vaccine Analytical Research and Development at Merck, and Chris Heger, Senior Manager, Applications Science at ProteinSimple.
- Watch Advances in Cell and Gene Therapy Analytics
Which system is right for me?
Source: Bio-Techne
| Platform Specifications |
Simple Western |
Simple Plex |
Maurice |
| Main Sample Type |
Virus and tissue lysates, supernatants |
Virus lysates, supernatants, and purified virus preparation |
Virus preparation (>70 % pure) |
| Assay Type |
CE-SDS/cIEF + immunoassay |
Sandwich ELISA |
CE-SDS or icIEF |
| Detection Modes |
Chemiluminescence, Fluorescence, Total protein stain |
Fluorescence |
Absorbance & Native Fluorescence |
| Sample Volume |
3 μL |
25 μL |
50 μL |
| Assay Development |
Single antibody — open platform |
None — Fully validated kits |
No antibody — open platform |
| Quantitative |
Yes |
Yes |
Yes |
| Size or Charge Separation |
Yes |
|
Yes |
| Dynamic Range |
3 to 4 logs |
3 to 5 logs |
3 logs |
| Detection Range (GC or VP/mL) |
109 – 1012 |
106 – 1010 |
1011 – 1014 |
| Runtime |
<3 hrs, <5 hrs with RePlex™ |
90 mins |
5 – 35 mins with CE-SDS, 10 – 15 mins with icIEF
|
Which systems address vector CQAs?
Source: Bio-Techne
| Critical Quality Attribute |
Attribute Detail |
Simple Western |
Simple Plex |
Maurice |
| Identity |
Is my virus present in the sample? |
Yes |
Yes |
Yes |
| Identity |
Am I confident it is the right virus? |
Yes |
Yes |
Yes |
| Potency |
Physical viral titer |
Yes |
Yes |
|
| Potency |
Functional viral titer |
Yes |
|
|
| Potency |
Protein Expression Potency |
Yes |
Yes |
|
| Potency |
Capsid protein ratio (VP ratios) |
Yes |
|
Yes |
| Purity |
Process-related impurities |
Yes |
Yes |
Yes |
| Purity |
Host cell-related impurities |
Yes |
Yes |
Yes |
| Purity |
Capsid Content (Empty/Full) |
Yes |
|
Yes |
| Stability |
Aggregate formation |
|
|
MFI |
| Stability |
Product stability |
Yes |
|
Yes |
About Bio-Techne
Bio-Techne Corporation is a leading developer and manufacturer of high-quality purified proteins - notably cytokines and growth factors, antibodies, immunoassays, as well as biologically active small molecule compounds - which are sold to biomedical researchers and clinical research laboratories; these operations constitute the core Biotech Division, headquartered in Minneapolis, Minnesota.
The Protein Platform Division manufactures innovative protein analysis tools under the ProteinSimple brand name that greatly automate western blotting and immunoassay practices. The Diagnostics Division manufactures FDA-regulated controls, calibrators, blood gas, and clinical chemistry controls for OEM customers and clinical customers. Bio-Techne products assist scientific investigations into biological processes and the nature and progress of specific diseases. They aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
With thousands of products in its portfolio, Bio-Techne generated approximately $563 million in net sales in fiscal 2017 and has approximately 1,800 employees worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.