Sponsored Content by Bio-TechneReviewed by Louis CastelJan 11 2024
Recent advancements and achievements in viral vector gene therapy underscore the importance of evaluating the critical quality attributes (CQAs) of the delivery systems being utilized in the process.
Adeno-associated viruses (AAVs) and Lentiviral vectors (LVVs) are regarded as the main platforms for gene delivery. The primary CQAs for this viral gene delivery system are identity and purity.
Previously, multiple platforms including SDS-PAGE, PCR, and ELISA were required to confirm purity and investigate serotypes; however, these attributes can now be efficiently, easily, and reliably analyzed with Maurice CE-SDS.
Maurice CE-SDS contains two different CE-SDS cartridges, Turbo CE-SDS and CE-SDS PLUS, which provide unique advantages during different development stages. Turbo CE-SDS is suited to high-throughput analysis and is the perfect choice for the discovery process. CE-SDS PLUS delivers higher resolution, making it more suitable for analytical purposes and QC release testing.
In this study, four AAV serotypes and AAV9 packaged with different transgenes were analyzed, using both Turbo CE-SDS and CE-SDS PLUS cartridges. The results provided comparable viral protein ratios (VP3:VP2: VP1), relative migration times, peak profiles, and excellent reproducibility. The level of impurities present in the samples was also detected and quantified.
The complex peak profile of LVV was also successfully resolved using the Turbo CE-SDS cartridge, producing an RSD of 1.92 % (9 sample injections from 3 different preparations). The data shows that Maurice CE-SDS can support viral vector characterization from discovery to GMP release.
Methods
Image Credit: Bio-Techne
Sample preparation
Cold acetone (4x the sample volume) was introduced to 20 µL of the SF9-derived AAV sample (Virovek, 2 x 1013 vg/mL) and vortexed briefly. The sample was subsequently kept at -20 °C for an hour.
The sample was then centrifuged at room temperature for 10 minutes at 15000 xg to pellet the proteins. The resulting supernatant was carefully removed, and the precipitate was left to dry for 5 minutes.
CE-SDS PLUS method
The precipitate was dissolved in 20 µL of the CE-SDS PLUS buffer and vortexed. To facilitate denaturation, 0.7 M β-mercaptoethanol (β-ME) was added to the buffer and incubated at 70 °C for 10 minutes.
The sample was then cooled on ice for 5 minutes and spun in a micro-centrifuge. Distilled water was added to bring the final volume to 50 µL or 100 µL for CE-SDS PLUS and Turbo CE-SDS, respectively.
For Turbo CE-SDS analysis, samples were injected for 8 seconds at 3500 V and separated for 8 minutes at 4200 V. For CE-SDS PLUS analysis, samples were injected for 20 seconds at 4600 V and separated for 35 minutes at 5750 V. All data were analyzed with Compass for iCE software.
Results
Identifying multiple stereotypes
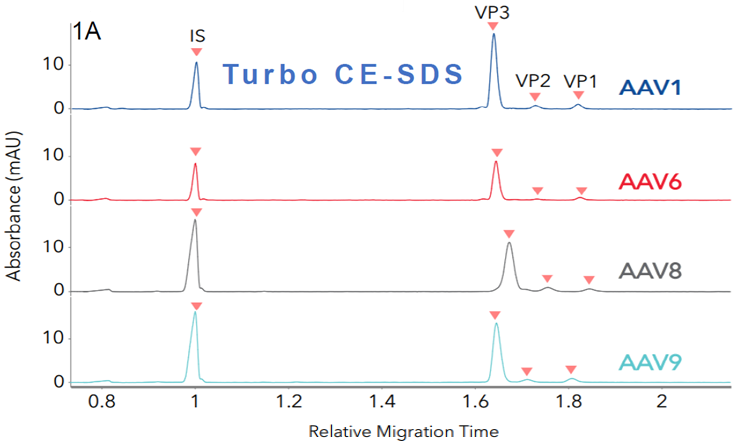
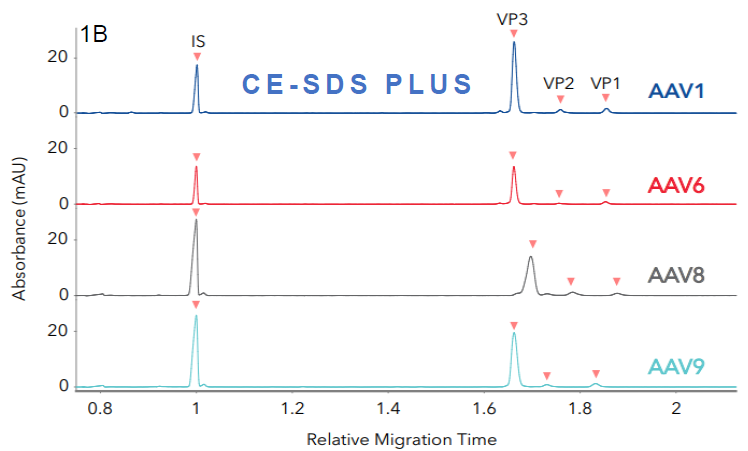
Figure 1. Identifying different AAV serotypes with Maurice CE-SDS. Four different AAV serotypes were analyzed with A. Turbo CE-SDS and B. CE-SDS PLUS cartridges. FIGURES 1A and 1B show stacked profiles of the four serotypes (AAV1, 6, 8, and AAV9) resulting from each cartridge. Image Credit: Bio-Techne
Source: Bio-Techne
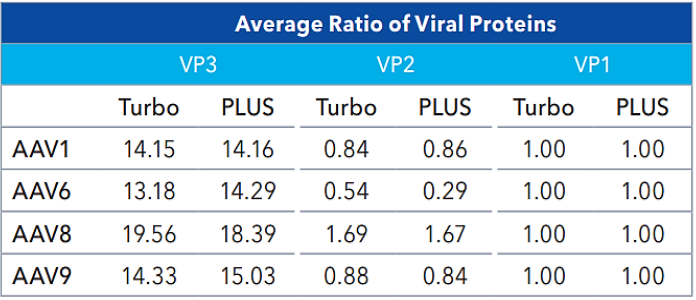
The average ratios of viral proteins (VP1, VP2, and VP3) exhibit comparability between the two methods, as shown in Table 1. Similar peak profiles and relative migration times between both cartridges were observed for each serotype, further highlighting the comparability of the two methods. These results demonstrate that the Turbo CE-SDS and CE-SDS PLUS methods effectively function as identity assays for AAV vectors.
Analyzing AAV9 with different transgenes
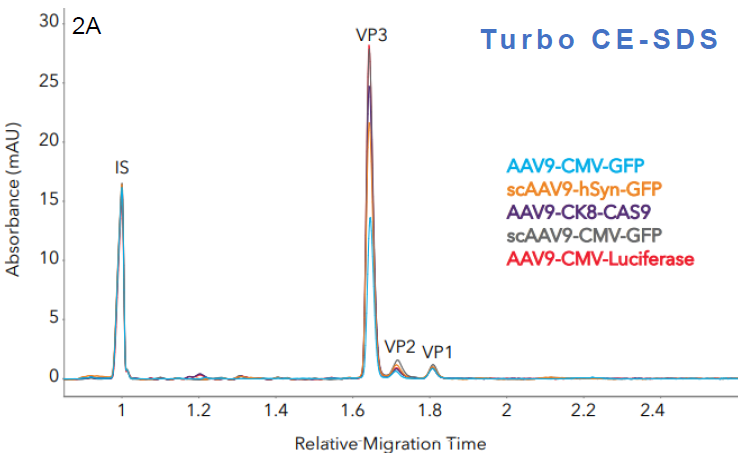
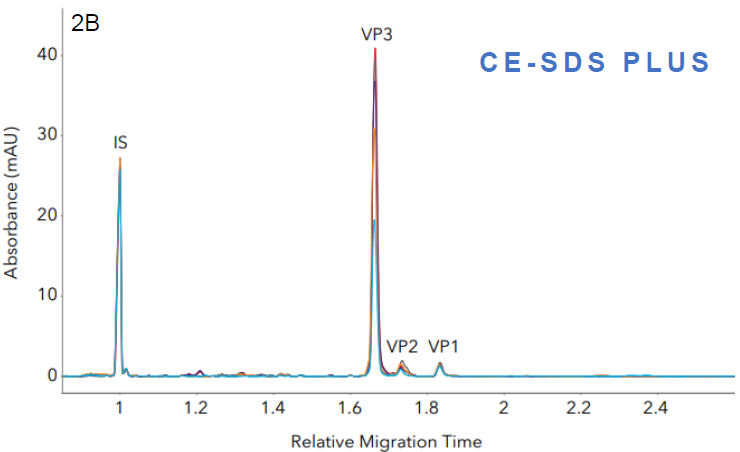
Figure 2. Measuring the capsid protein ratio of AAVs with Maurice CE-SDS. Five AAV samples with different transgenes were analyzed with A. Turbo CE-SDS and B. CE-SDS PLUS. Different sample profiles were clearly captured by both methods, from which the respective capsid protein ratios were measured. In both cases, the peak profiles and viral protein ratios remain comparable. Image Credit: Bio-Techne
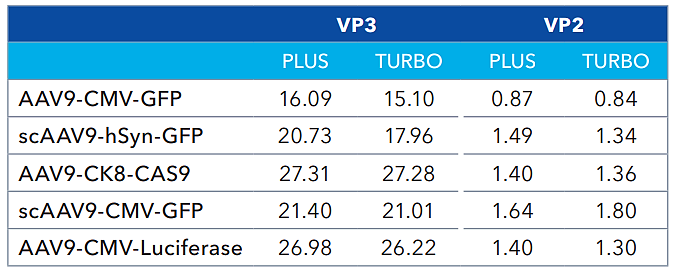
Capsid protein ratios (Turbo vs PLUS). Source: Bio-Techne
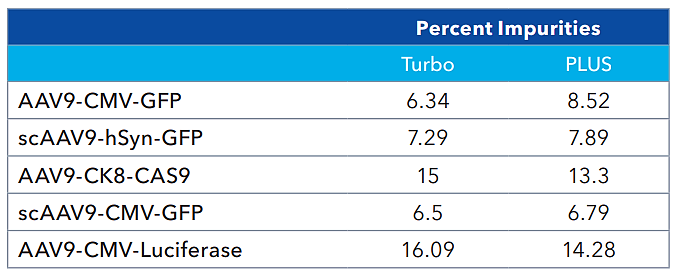
Impurity measurements
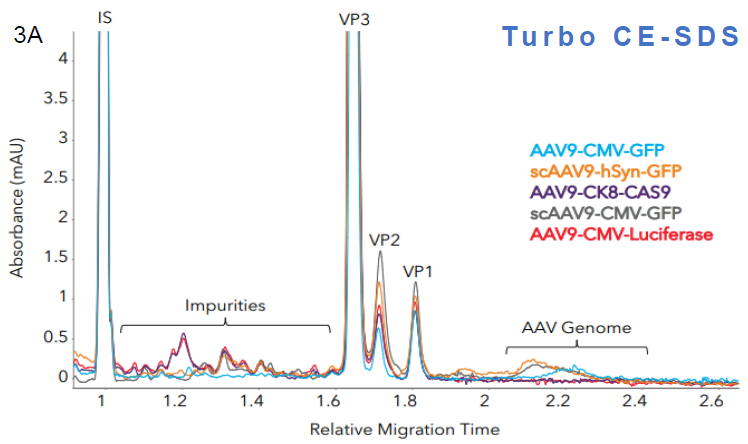
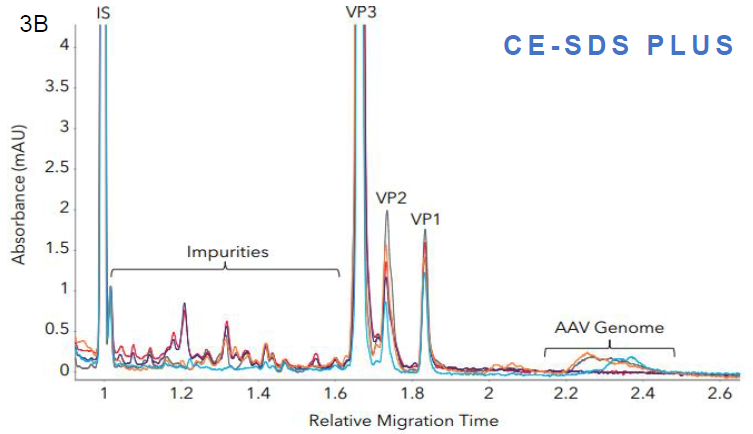
Figure 3. Measuring impurities in different AAV samples with Maurice CE-SDS. Five AAV samples with different inserts were analyzed with A. Turbo CE-SDS and B. CE-SDS PLUS. Both methods were able to detect and quantify the level of impurities and the AAV genome present in the samples comparably. Image Credit: Bio-Techne
Impurity quantitation (Turbo vs PLUS). Source: Bio-Techne
Reproducibility
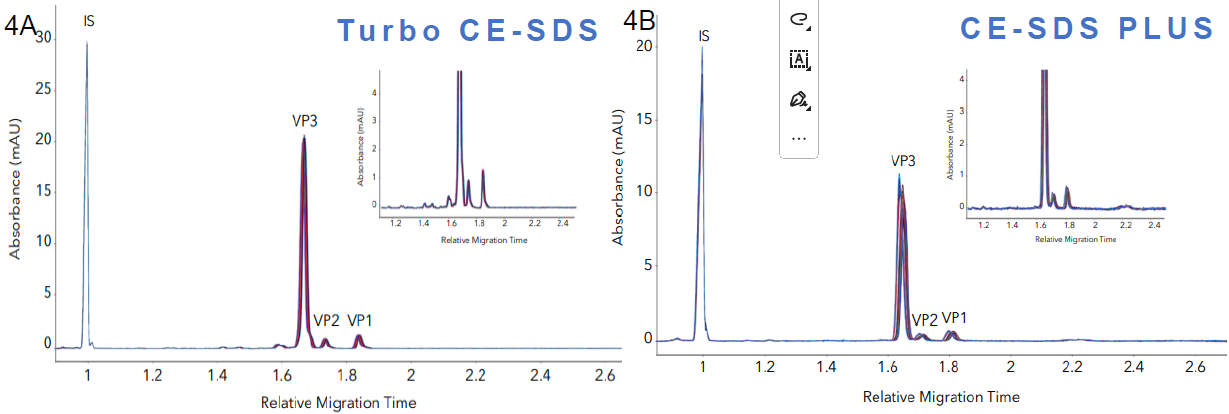
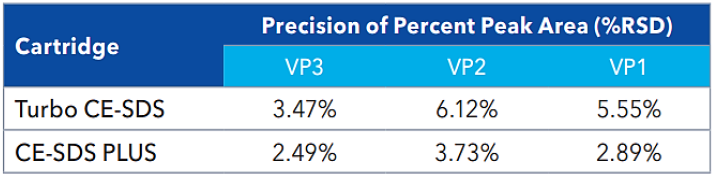
Figure 4. Method reproducibility of Turbo CE-SDS & PLUS. A. Overlaid profiles of 93 AAV sample injections show excellent reproducibility of the method. B. Overlaid profiles of 45 AAV sample injections demonstrate the high reproducibility of the method. Image Credit: Bio-Techne
Source: Bio-Techne
LVV reproducibility
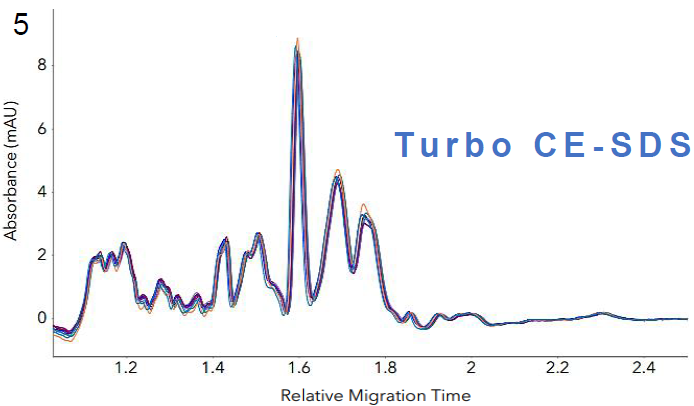
Figure 5. Intra-assay reproducibility of the Turbo CE-SDS cartridge analyzing LVV samples. Profiles of nine sample injections from three different preparations demonstrate
high reproducibility of the method, with an RSD value of 1.92 % for the total area. Image Credit: Bio-Techne
Conclusions
Both Turbo CE-SDS and CE-SDS PLUS methods effectively characterized the identity and purity of AAV samples, showcasing outstanding inter- and intra-assay reproducibility.
Both assays produced distinct profiles for various AVV stereotypes. The capsid protein ratios were measured, and the impurities in samples were easily detected. Crucially, both cartridges generated comparable data sets in all cases.
About Bio-Techne

Bio-Techne Corporation is a leading developer and manufacturer of high-quality purified proteins - notably cytokines and growth factors, antibodies, immunoassays, as well as biologically active small molecule compounds - which are sold to biomedical researchers and clinical research laboratories; these operations constitute the core Biotech Division, headquartered in Minneapolis, Minnesota.
The Protein Platform Division manufactures innovative protein analysis tools under the ProteinSimple brand name that greatly automate western blotting and immunoassay practices. The Diagnostics Division manufactures FDA-regulated controls, calibrators, blood gas, and clinical chemistry controls for OEM customers and clinical customers. Bio-Techne products assist scientific investigations into biological processes and the nature and progress of specific diseases. They aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
With thousands of products in its portfolio, Bio-Techne generated approximately $563 million in net sales in fiscal 2017 and has approximately 1,800 employees worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.