This poster was originally authored by John Vincent, Matthew Burnham, Gerrit Daubner, and Martin Main of the Medicines Discovery Catapult. It was presented at ELRIG Drug Discovery 2023.

Methods to rapidly determine ligand target engagement are valuable tools in drug discovery. Developing a straightforward plate‐based assay workflow allows compatibility with HTS settings. MDC has developed a proprietary lysate‐based chemical protein denaturation technology to confirm cellular protein target engagement by monitoring compound binding-induced stabilization in a simple, automation‐friendly, scalable, cost‐effective assay.
The Chemical Protein Stability Assay (CPSA) is a simplified single well, plate‐based workflow without plate transfer, suited to compound screening and hit finding, and compatible with multiple assay endpoints. The patent-pending technology does not require labeling a tracer ligand or modifying the target protein. Cell lysate fits well with large‐scale applications where confounding cellular permeability effects are not desirable at early hit ID while avoiding issues with large‐scale protein purification. The simplicity of the workflow and reagents reduces assay variability and produces consistent data. CPSA is broadly applicable to a wide range of cellular targets.
Comparison to established workflows
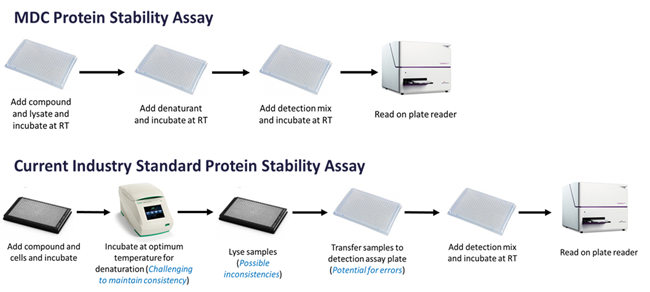
Figure 1. Image Credit: ELRIG (UK) Ltd.
The MDC CPSA is a proprietary simple, lysate‐based, single‐plate, mix-and-read assay able to measure target engagement by assessing protein stability when unfolded using a chemical denaturant. Thus, it is highly amenable to multi‐well plate compound screening. The assay has several advantages over existing assays that make it reliable and easy to use –
- Basic level of equipment allows accessibility to wide range of labs
- Relatively few steps makes it simple to run and allows scalability to HTS
- No high-temperature incubations
- No lysis step reduces inconsistencies
- No plate transfer increases application to use in HTS
- Use of lysate reduces variability that may be seen with cells
- Use of lysate removes need to generate isolated protein
- Inexpensive and cost‐effective
Proof of concept
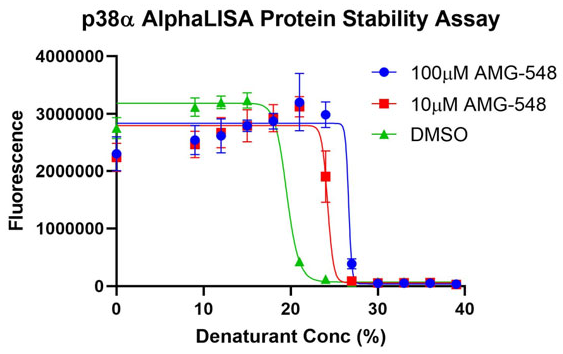
Figure 2. Image Credit: ELRIG (UK) Ltd.
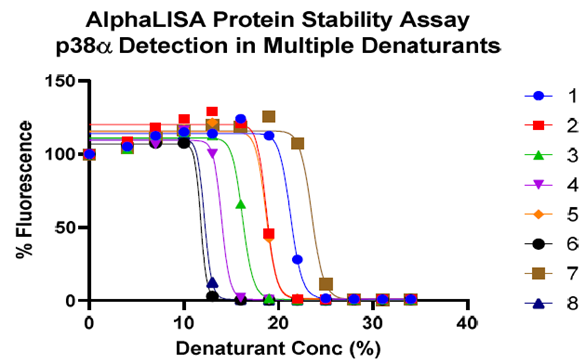
Figure 3. Image Credit: ELRIG (UK) Ltd.
Using HEK293 lysate as a source of protein, the compound AMG‐548 was shown to cause the stabilization of p38α MAPK when denatured using a chemical denaturant. Treatment with or without compound lead to a clear concentration‐dependent curve shift, detectable in a single‐well assay using an AlphaLISA® Immunoassay endpoint (Fig. 2). A range of denaturants can be used to build an optimum assay for the chosen target, allowing the unfolding of more stable proteins at less endpoint‐detrimental chemical concentrations (Fig. 3). CPSA is compatible with a range of endpoint technologies and a range of target proteins.
Compound screening and concordance
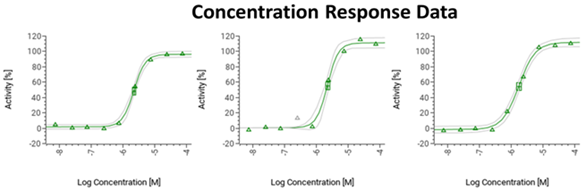
Figure 4. Image Credit: ELRIG (UK) Ltd.
Using a fixed amount of denaturant, the CPSA can generate dose-response curves that show a compound stabilisation effect (Fig. 4).
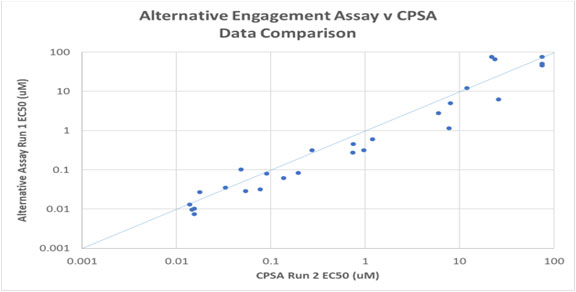
Figure 5. Image Credit: ELRIG (UK) Ltd.
A set of 23 compounds with a range of activities against p38α MAPK were tested in both the CPSA and an established alternative target engagement assay. The data generated from the two assays showed good agreement in rank order (Fig. 5).
Application to HTS single-point screening
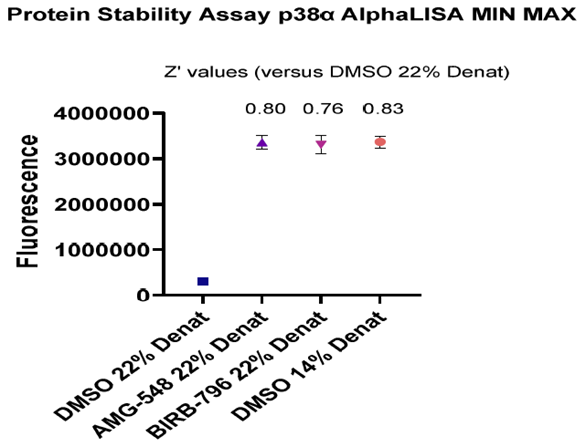
Figure 6. Image Credit: ELRIG (UK) Ltd.
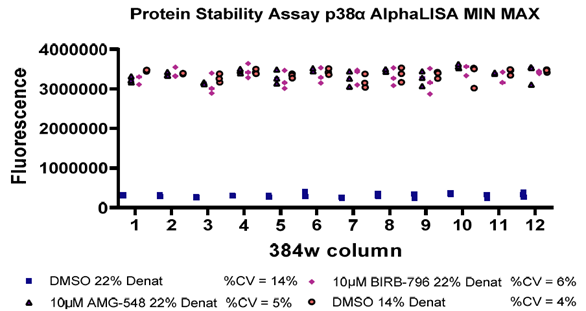
Figure 7. Image Credit: ELRIG (UK) Ltd.
A CPSA using the known inhibitors to p38α MAPK, AMG‐548, and BIRB 796 was run to generate Max/Min data to assess the assay application to single point HTS. The compounds demonstrated clear stabilization of p38α MAPK in the presence of a fixed denaturant concentration, and complete protein unfolding was achieved at the same denaturant concentration in the absence of the compound. A lower denaturant concentration was also tested in the absence of a compound, demonstrating that a maximal stabilization window had been achieved. Excellent Z values were generated (Fig.6), and good reproducibility of replicate data across the plate was seen, as shown in Fig. 6, which had good % CV values (Fig. 7).
Conclusions
MDC has developed a proprietary chemical protein stability assay (CPSA) that is comprised of a simple, cost‐effective workflow without transfer steps and applies to compound profiling and hit identification. The patent pending assay applies to a range of protein targets and can be configured using a range of protein detection technologies. The CPSA can generate high‐quality data in both dose-response and single-point format to detect the engagement of compounds with target proteins. Data using the CPSA is comparable to that generated using established alternative techniques and demonstrates the application of this technique to compound screening and Hit finding activities.
About John Vincent
John Vincent is a senior scientist with more than 25 years of experience in drug discovery roles, developing biochemical and cellular assays in a range of technologies and formats, utilizing an array of laboratory equipment and automation. 
John has a degree from the University of Nottingham and previously worked for 23 years at AstraZeneca in roles spanning microbiology, oncology, and high-throughput screening. In his role at the Medicines Discovery Catapult, John is responsible for the development and validation of robust cellular and biochemical in vitro assays to monitor target engagement and modulation. He also provides expertise in the areas of drug discovery, screening, and automation.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.