This poster was originally authored by Charlotte R Bell, Tania Katopodi, Yin Xin Ho, Emily Wright, Amy Cantrell, Nick Moore, Stewart Brown, Paul Farrington, Lorraine Mooney & Jane Kendrew Translational Oncology, Sygnature Discovery. It was presented at ELRIG Drug Discovery 2023.

Introduction
- Radiotherapy is a main treatment modality for multiple cancer types: ~50% all cancer patients receive radiotherapy as part of their treatment regimen1,2
- Irradiation (IR) of tumours can induce direct cytotoxic effects on cancer cells and modulate the tumour immune microenvironment (TIME)3,4
- Therapies which augment anti-cancer immune responses, such as immune checkpoint inhibitors (ICIs) targeting the PD-(L)1 axis, have revolutionised cancer treatment in recent years5
- Multiple clinical trials are evaluating combinations of radiotherapy + ICIs3
- Characterising the effects of IR + ICIs on the TIME and tumour growth is therefore valuable to inform preclinical drug discovery research
Methods
- MC38 colorectal carcinoma cells were implanted subcutaneously into female C57BL/6 mice and tumour growth was monitored 3 times per week using callipers
- Animals were randomised into treatment groups once tumours reached ~0.1 cm3
- Targeted X-rays were administered using an Xstrahl CIX3 cabinet irradiator. Animals were shielded using lead panels, with only the tumour exposed for localised IR treatment
- ICIs anti-PD-1 (RMP1-14) and anti-PD-L1 (10F.9G2) were purchased from BioXCell and dosed intraperitoneally (IP) twice weekly at a dose of 10 mg/kg
- Tumours analysed by flow cytometry were excised, digested into a single cell suspension and stained with the below antibody panel. Samples were acquired on an Attune NxT Flow Cytometer (ThermoFisher Scientific)
Source: ELRIG (UK) Ltd.
| Channel |
Marker |
Fluorochrome |
| VL-1 |
PD-1 |
BV421 |
| VL-2 |
live/dead |
efluor506 |
| VL-4 |
CD4 |
BV711 |
| BL-1 |
CD8 |
FITC |
| YL-1 |
CD11b |
PE |
| YL-2 |
CD62L |
PE-CF594 |
| YL-3 |
CD45 |
PE-Cy5.5 |
| YL-4 |
FOXP3* |
PE-Cy7 |
| RL-1 |
F4/80 |
Alexa Fluor 647 |
| RL-2 |
CD3 |
Alexa Fluor 700 |
| RL-3 |
CD44 |
APC-efluor 780 |
*intracellular antibody
1. Fractionated IR leads to tumour growth inhibition in the MC38 tumour model
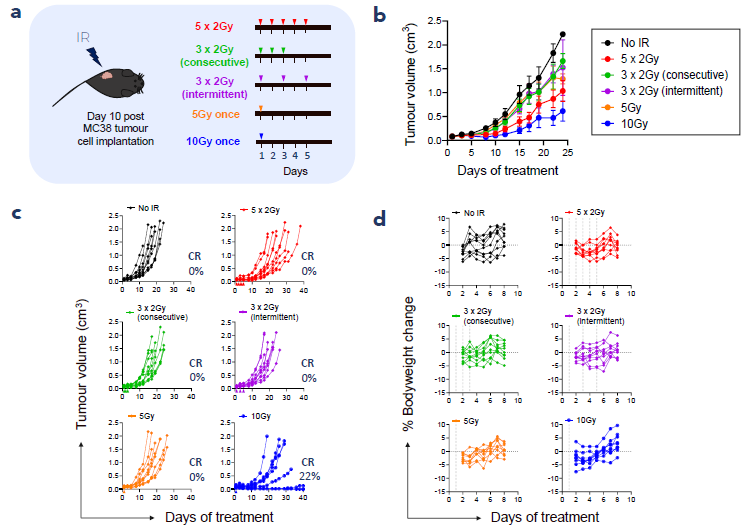
Figure 1. Fractionated IR is efficacious and well tolerated in C57BL/6 MC38 tumour-bearing mice. (a) Study schematic, (b) mean tumour volume (±SEM), (c) individual tumour volume, (d) individual % change in bodyweight over one week of treatment. n = 9 or 10 per group, CR = complete response. Image Credit: ELRIG (UK) Ltd.
2. IR + ICI combinations enhance tumour growth control
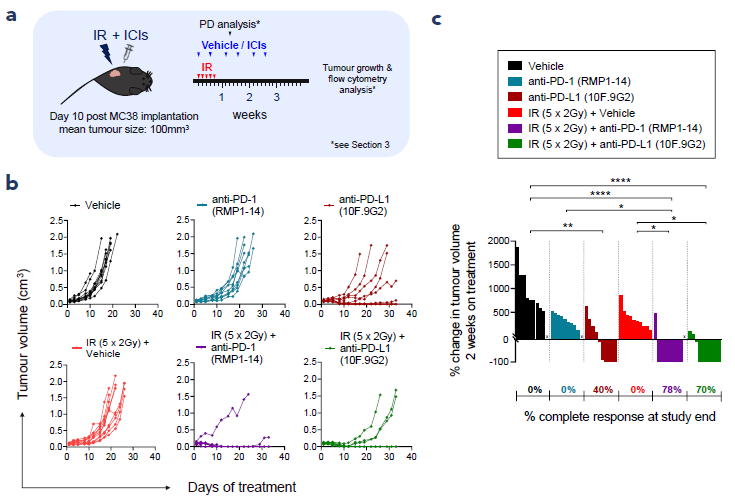
Figure 2. Combinations of fractionated IR with ICIs have improved efficacy compared with the monotherapies. (a) Study schematic, (b) individual animal tumour volume over time, (c) percent change in tumour volume 2 weeks on treatment. n = 10 per group, *p<0.05, **p<0.01, ****p<0.0001 as determined by Kruskal-Wallis with Dunn’s multiple comparisons test. Image Credit: ELRIG (UK) Ltd.
3. IR + ICI combinations modulate the intratumoural immune infiltrate
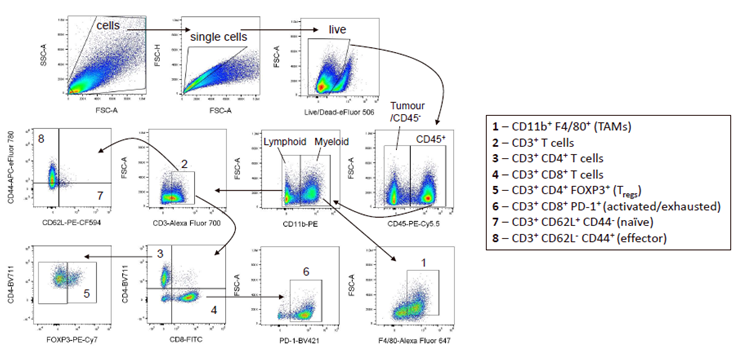
Figure 3. Flow cytometry gating strategy on MC38 tumours. Image Credit: ELRIG (UK) Ltd.
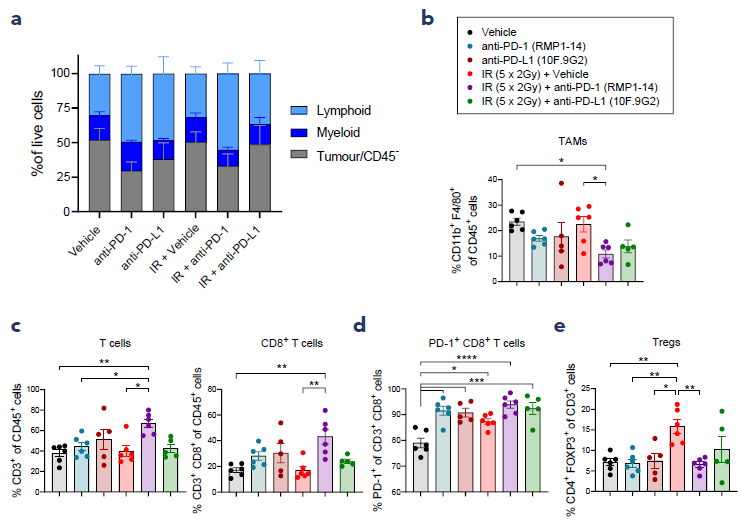
Figure 4. Changes to the intratumoural immune composition induced by IR + ICI combinations on day 9 of treatment. (a) Frequency of lymphoid and myeloid CD45+ cells and tumour/CD45- cells as a percentage of live cells, (b) frequency of tumour associated macrophages (TAMs) as a percentage of CD45+ cells, (c) frequency of CD3+ T cells and CD8+ T cells as a percentage of CD45+ cells, (d) frequency of PD-1+ cells as a percentage of CD8+ T cells, (e) frequency of regulatory T cells (Tregs) as a percentage of CD3+ T cells. Bars represent mean ±SEM, n = 5 or 6 per group, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 as determined by one-way ANOVA with Tukey’s multiple comparisons test. Image Credit: ELRIG (UK) Ltd.
Summary
- Fractionated radiotherapy and immune checkpoint inhibitor combinations show significantly improved efficacy in the MC38 tumour model
- The combination of fractionated radiotherapy with anti-PD-(L)1 immune checkpoint inhibitors impacts the tumour immune microenvironment
- These data demonstrate the MC38 tumour model is a valuable tool for immunooncology preclinical drug discovery research
References
- Baskar R, Lee KA, Yeo R, Yeoh KW, 2012. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9(3), pp.193-199
- Public Health England, 2020. [Online]. Chemotherapy, Radiotherapy and Surgical Tumour Resections in England. Available at: https://www.gov.uk/government/statistics/chemotherapy-radiotherapy-and-surgicaltumour-resections-in-england/chemotherapyradiotherapy-and-surgical-tumourresections-in-england (accessed 02.05.23)
- McLaughlin, M, Patin, EC, Pedersen, M, Wilkins, A, Dillon, MT, Melcher, AA, Harrington, KJ, 2020. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20(4), pp.203–217
- Rodriguez-Ruiz, ME, Vitale, I, Harrington, KJ, Melero, I, Galluzzi, L, 2020. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21(2), pp.120–134
- Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A et al., 2023. Immune checkpoint therapy - current perspectives and future directions. Cell 186(8), pp.1652-1669
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.