Sponsored Content by TA InstrumentsReviewed by Maria OsipovaOct 10 2024
Short-term thermal stability testing is vital for analyzing a compound’s resistance to thermal stress, which is crucial for estimating shelf life and preserving efficacy. Differential Scanning Calorimetry (DSC) is a strategy utilized to determine the structure of antibodies, but it is difficult to define formulation concentration samples using conventional tools.
These difficulties originate from using an irreplaceable sample cell, which requires extensive cleaning steps to guard against sample carryover between scans. The fixed sample cell further constrains the upper concentration range because highly concentrated protein has a greater tendency to clog the cell when heated. At best, a clogged cell necessitates lengthy, harsh cleaning to regenerate.
At worst, a cell can be permanently contaminated. Samples, therefore, require dilution from their formulation concentrations. Concentration can impact protein thermal stability, so it is essential to test different formulation strengths to understand how potential drugs remain stable in storage.
The TA Instruments Rapid Screening-Differential Scanning Calorimeter (RS-DSC) is an innovative differential scanning microcalorimetric technology advancement. Unlike other DSCs, it does not need samples to be diluted. It is specially engineered to handle high-concentration biologic drug formulations in low-volume and disposable microfluidic chips.
This technological integration removes the requirement for repeated cleaning of the tool measurement cell between runs, saving time, minimizing contamination risk, and allowing for more dependable readings. A specimen can be readied, sealed, and made available for evaluation in under a minute, requiring minimal volumes for accurate analysis.
The TA Instruments RS-DSC is a novel platform for high-throughput thermal stability screening in the biopharmaceutical industry. It revolutionizes the field by concurrently evaluating 24 samples. It delivers thorough physicochemical insights via thermal analysis, which can accelerate the development of pharmaceuticals and streamline their introduction to market.
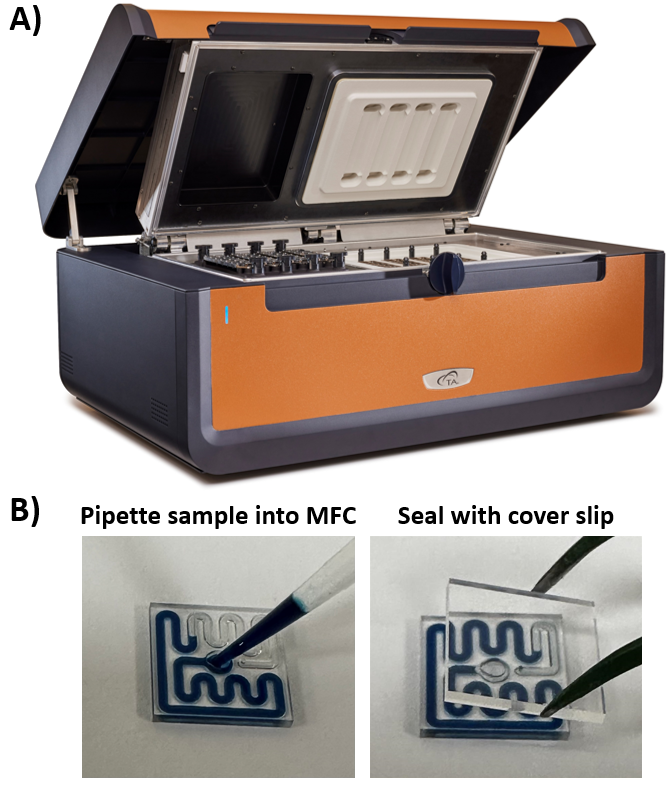
Figure 1. A) TA Instruments RS-DSC. B) Sample is prepared by pipetting protein solution into MFC and sealing with a glass cover slip. Image Credit: TA Instruments
Experiments and methods
Herceptin® trastuzumab was formulated following package guidelines in histidine formulation buffer (18.4 mg/mL trehalose dihydrate, 0.08 mg/mL polysorbate 20, 0.49 mg/mL histidine HCl, 0.32 mg/mL histidine, pH 6.0) at 21 mg/mL and maintained at 4 °C until usage.
For evaluation in phosphate-buffered saline (PBS), the antibody was buffer exchanged via an Amicon™ Ultra 10kDa molecular weight cut-off centrifugal spin filter into PBS comprised of Gibco™ DPBS with 1 mM EDTA at pH 7.4, calibrated to the preferred concentration, and examined in triplicate.
Chicken egg white lysozyme, utilized for analyzing high-concentration protein solutions, was acquired from Sigma Aldrich (L6876) and prepared in glycine buffer (0.1 M glycine, pH 2.5). Protein samples were concentrated to 330 mg/mL via an Amicon Ultra 3kDa molecular weight cut-off centrifugal spin filter.
The concentrated solution produced dilutions. Immediately following protein concentration preparation, tests were executed in triplicate using the TA Instruments RS-DSC.
Protein samples were directly pipetted into the disposable glass microfluidic chips (MFCs) with a channel engineered to have 11 μL of liquid sample. After adding the protein solution, the MFCs were sealed using an adhesive-backed glass coverslip to include the specimen while increasing the temperature to 100 °C. They were ready to be put into the TA Instruments RS-DSC (as shown in Figure 1).
Fully assembled MFCs were then put onto the sample side of both twin calorimeters. A reusable polyetheretherketone chip remained on the reference side. As many as 24 samples can be run concurrently with a maximum temperature range of 20–100 °C and preset scan rates of 1 or 2 °C/min. By removing the requirement for cleaning between scans, as many as 96 samples can be assessed in an ordinary workday.
The TA Instruments RS-DSC, run using TA Instruments RSDSCRun software, was configured to equilibrate at the starting temperature for 1800 seconds before the beginning of every scan. Every sample was run in triplicate and scanned at 1 or 2 °C/min across the 20 to 100 °C temperature range.
To guarantee precision across all 24 calorimeters, the tool was primarily adjusted and validated with dipalmitoylphosphatidylcholine, with an accepted Tmax shift for each calorimeter under 0.2 °C from the expected literature value.1
Data was processed utilizing NanoAnalyze™ Software (v4.0.0). New software features enable automatic denaturation midpoint temperature (Tmax) detection and comparison of as many as 96 thermograms. NanoAnalyze Software v4.0.0 has an innovative software algorithm, RapidDSC, that automatically detects Tmax in DSC thermograms by looking for peaks across the specified data range.
An automatic baseline is applied across the peak to refine the Tmax temperature for enhanced precision. Automatic peak detection properties show up to three peaks over the temperature range of interest.
The automation properties can be easily adjusted in the “Baseline and Tmax Editor” pop-out window to decrease the detection window, choose a range for peak identification, or calibrate the automatic baseline and Tmax. Tabulated data from Tmax automation can be transferred for smooth comparison or viewed in the Tmax visualization and overlay tabs in RapidDSC.
Results and discussion
TA instruments RS-DSC limit of detection
With low sample volume needs, understanding the concentration limits required for data sensitivity and precision is essential. More rapid scan rates lead to greater signal intensity, which can help evaluate protein concentrations at or close to the detection limit.
To comprehend the concentration criteria for a multi-transition protein, the antibody Herceptin trastuzumab was altered to different concentrations in PBS and analyzed in triplicate at a scan rate of 2 °C/min (Figure 2 and Table 1).
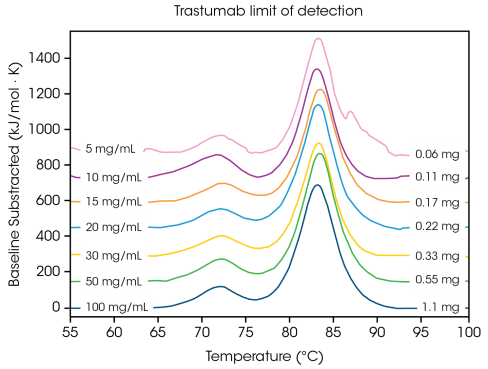
Figure 2. Representative DSC scans of Herceptin Trastuzumab (0.06 mg – 1.1 mg per scan), collected in triplicate at 2 °C/min and normalized to molar heat capacity for direct comparison. Image Credit: TA Instruments
Table 1. Transition temperatures and reproducibility across limit of detection screen. Mean ± SD, n = 3. Source: TA Instruments
| Conc. |
Tmax,1 (°C) |
Tmax,2 (°C) |
| 5 mg/mL |
72.57 ± 0.52 |
83.06 ± 0.09 |
| 10 mg/mL |
71.83 ± 0.29 |
82.98 ± 0.05 |
| 15 mg/mL |
72.13 ± 0.23 |
83.17 ± 0.11 |
| 20 mg/mL |
71.88 ± 0.01 |
82.99 ± 0.08 |
| 30 mg/mL |
71.91 ± 0.12 |
83.10 ± 0.03 |
| 50 mg/mL |
71.93 ± 0.08 |
83.20 ± 0.02 |
| 100 mg/mL |
71.85 ± 0.01 |
83.01 ± 0.01 |
At a concentration of 100 mg/mL, two transitions are evident, corresponding to both the unfolding of the CH2 domain at an average Tmax,1 of 71.85 °C, and the combined Fab and CH3 domain at an average Tmax,2 of 83.01 °C.2 No considerable alteration in stability is noted as protein concentration is reduced, and superb reproducibility across calorimeters is noted.
At 20 mg/mL Herceptin trastuzumab, the magnitude of the CH2 unfolding transition is considerably smaller, but excellent reproducibility is noted across triplicate samples (as shown in Figure 3.). Under 20 mg/mL, evaluating the smaller CH2 unfolding domain becomes more difficult, while greater variability in the identified Tmax,1 is noted. Larger unfolding events, like the combined Fab and CH3 domains (Tmax,2) are more easily identified, even at concentrations as low as 5 mg/mL.
Like all DSC tools, sharper, narrower peaks yield the highest reproducibility. The limit of detection is sample-dependent and can differ depending on protein complexity and the solution environment.
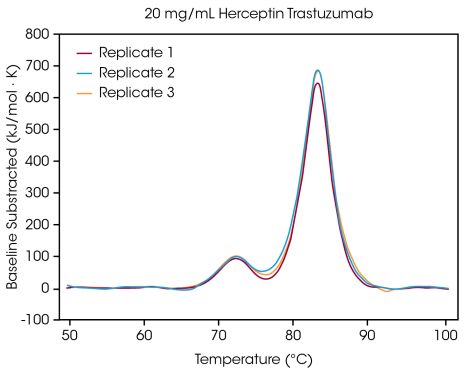
Figure 3. Triplicate thermograms of Herceptin Trastuzumab in PBS buffer at 20 mg/mL. Image Credit: TA Instruments
Investigation of concentration dependence on thermal stability
The TA Instruments RS-DSC is specially engineered to handle high-concentration biologic drug samples, particularly antibody drugs and antibody-drug conjugates. With the increasing success of antibody treatments, the pharmaceutical industry is increasingly interested in dosage forms of high concentration that allow for both subcutaneous and ocular therapeutic delivery.
Concentrations of 50 to 150 mg/mL antibodies are common, with some being as high as 200+ mg/mL.3 Formulating proteins at high concentrations can heighten susceptibility to physical instability.4,5 Research has also demonstrated improved thermal stability at higher concentrations in some cases.6
Understanding thermal unfolding and response to the solution environment at the formulation concentration of interest is, therefore, key to preventing pharmaceutical liability.
The TA Instruments RS-DSC is engineered to keep protein samples in disposable microfluidic chips. Conventional microcalorimetric tools contain a fixed sample cell that needs to be cleaned between experiments.
High-concentration samples vulnerable to precipitation or gelation throughout the unfolding process can be difficult to clean and may even damage the cell. In addition to enhancing ease of operation and eliminating lengthy cleaning protocols, the disposable microfluidic chips utilized in the TA Instruments RS-DSC permit the risk-free testing of high-concentration protein samples.
Chicken egg white lysozyme from 30 to 330 mg/mL in glycine buffer (Figure 4 and Table 2) was analyzed to show the capability to screen high-concentration protein samples and display the value of testing at the preferred formulation concentration.
Lysozyme is often used as a DSC reference test sample with a simple single transition thermogram at low concentrations (~1 mg/ mL). By evaluating protein concentrations up to 100 times higher, TA Instruments observed a concentration dependence on lysozyme stability.
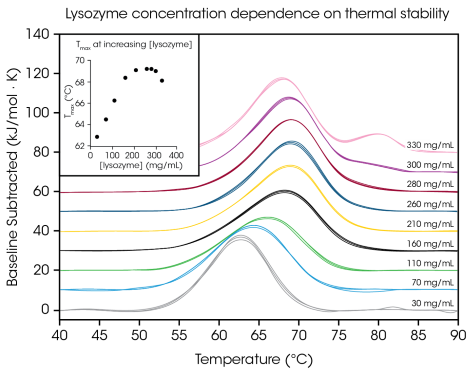
Figure 4. Short term thermal stability of lysozyme at concentrations from 30 to 330 mg/mL in glycine buffer, data in triplicate. Inset: Average Tmax relative to lysozyme concentration. Image Credit: TA Instruments
Table 2. Transition temperatures and reproducibility across the screen of lysozyme concentration dependence. Mean ± SD, n = 3. Source: TA Instruments
| Conc. |
Tmax (°C) |
| 330 mg/mL |
68.14 ± 0.05 |
| 300 mg/mL |
69.00 ± 0.04 |
| 280 mg/mL |
69.22 ± 0.04 |
| 260 mg/mL |
69.19 ± 0.09 |
| 210 mg/mL |
69.08 ± 0.06 |
| 160 mg/mL |
68.39 ± 0.04 |
| 110 mg/mL |
66.27 ± 0.09 |
| 70 mg/mL |
64.68 ± 0.05 |
| 30 mg/mL |
62.81 ± 0.06 |
At the lowest concentration tested, 30 mg/mL, the Tmax of lysozyme was assessed to be 62.81 °C. As concentration increased to 160 mg/mL, stability increased linearly (see Figure 4 inset) to a Tmax of 68.39 °C. From 210 – 280 mg/mL, the thermal stability plateaued at a Tmax of roughly 69.2 °C.
At a concentration of 300 mg/mL, a shoulder at the tail of the main transition was noted at approximately 80 °C, and the general stability of the main transition marginally decreased with a Tmax of 69 °C.
At the highest concentration of 330 mg/mL, the thermal stability of the main transition Tmax,1 further decreased to 68.14 °C, and another peak is noted as having a Tmax,2 of 80.28 ± 0.04 °C. With the capacity for high-concentration testing, the TA Instruments RS-DSC enables the observation of higher-order structure(s) formation at lysozyme concentrations exceeding ~300 mg/mL.
In addition to showing the capability to obtain high-quality data at high protein concentrations, this research demonstrates the value of testing biologic pharmaceuticals at the concentration of interest since dilution may change the observed protein stability.
Conclusions
Conventional single-sample DSC technology is highly precise but requires large sample volumes and can be a time-intensive strategy in drug development. The TA Instruments RS-DSC allows for higher-throughput thermal stability testing via concurrent evaluation of as many as 24 samples.
A simpler workflow, a higher data acquisition rate via parallel testing, and more rapid analysis via automatic peak detection algorithms greatly improve time to results. In this article, TA Instruments demonstrated precise, reproducible analysis of complex-multiple transition antibody samples as low as 20 mg/mL.
The more rapid scan rate of 2 °C/min results in increased amplitude signals, which can help evaluate samples at or close to the detection limit. Examining high-concentration lysozyme solutions up to 330 mg/mL uncovers unexpected concentration reliance on thermal stability and underlines the value of analyzing the drug-product concentration of interest.
The TA Instruments RS-DSC offers a novel platform for identifying biotherapeutic stability, a valuable variable in understanding resilience to thermal stress, product quality, and supporting regulatory approval.
References and further reading
- “Standard Practice for Calibration of Fixed-Cell Differential Scanning Calorimeters,” ASTM E2603-15, 2023.
- K. J. Arlotta, A. V. Gandhi, H.-N. Chen, C. S. Nervig, J. F. Carpenter and S. C. Owen, “In-Depth Comparison of Lysine-Based Antibody-Drug Conjugates Prepared on Solid Support Versus in Solution,” Antibodies, vol. 7, p. 6, 2018.
- R. G. Strickley and W. J. Lambert, “A review of formulations of commercially available antibodies,” Journal of Pharmaceutical Sciences, vol. 110, pp. 2590-2608, 2021.
- J. Zarzar, T. Khan, M. Bhagawati, B. Weiche, J. Syndow-Andersen and S. Alavattam, “High concentration formulation developability approaches and considerations,” MAbs, vol. 15, pp. 1-13, 2023.
- S. J. Shire, Z. Shahrokh and J. Liu, “Challenges in the development of high protein concentration formulations,” Journal of Pharmaceutical Sciences, vol. 93, pp. 1390-1402, 2004.
- C. Zhang, J. W. Bye, L. H. Lui, H. Zhang, J. Hales, S. Brocchini, R. A. Curtis and P. A. Dalby, “Enhanced Thermal Stability and Reduced Aggregation in an Antibody Fab Fragment at Elevated Concentrations,” Molecular Pharmaceutics, vol. 20, pp. 2650-2661, 2023.
About TA Instruments
TA Instruments designs and manufactures the world’s highest performing thermal analysis, rheometer, microcalorimeter, and mechanical test systems by focusing on four fundamental measurements: temperature, force, weight, and displacement.
No other company or combination of companies sells as many of these systems as TA Instruments. Our products are used on a daily basis in the world’s most prestigious companies, universities and government laboratories.
Headquartered in New Castle, Delaware, TA manufactures at 7 different locations in the United States and Europe, and has direct sales and support staff in 24 countries and 5 continents.
TA Instruments has long been recognized for the technical competence and professionalism of our sales force. We are consistently ranked #1 by third party surveys for our responsive and knowledgeable support, the hallmark of our company.
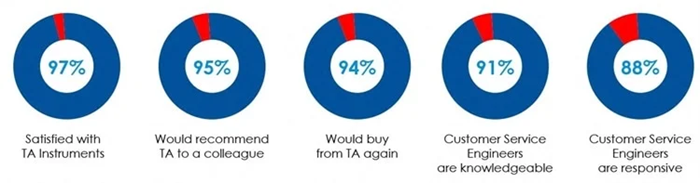
TA Instruments reputation for high performance products, quality manufacturing, and unbeatable after sales support is why more customers recommend TA products to their colleagues, compared to any other vendor. Here is what TA customers say about TA in a recent survey:
TA Instruments – Product Guide

Thermal Analyzers - Differential Scanning Calorimetry, Thermogravimetric Analyzers, Simultaneous Thermal Analyzers, Vapor Sorption Analyzers, Dynamic Mechanical Analyzers, Thermomechanical Analyzers

Rheometers - Discovery Hybrid Rheometers, ARES-G2 Rheometers, Dynamic Mechanical Analyzers, Rubber Rheometers

Microcalorimeters - Differential Scanning Calorimetry, Isothermal Titration Calorimetry, Isothermal Calorimetry

Dilatometers - Horizontal Dilatometers, High Resolution Horizontal Dilatometers, Vertical Dilatometers, Quenching Dilatometers, Optical Dilatometers, Heating Microscopes, Optical Platform

Thermal Conductivity Meters - Insulation & Building Materials, Heat Flow Meters, Guarded Hot Plate

Laser and Xenon Flash Analyzers

ElectroForce Mechanical Test Instruments - Load Frames, High Force Dynamic Mechanical Analyzers, TestBench and Planar Biaxial Testing, Cardiovascular Testing, Tissue Engineering

Rubber Testing Instruments - RPA, MDR, Mooney, Density & Hardness Testers, Sample Cutter, Mechanical Fatigue Testing/DMA

Primary Activity
Service Provider
Thermal analysis, rheometer and microcalorimetry supplier.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.