Sponsored Content by TA InstrumentsReviewed by Maria OsipovaOct 10 2024
Understanding antibodies’ structure-function relationship, whether because of sequence modifications or changes in solution conditions, is crucial to understanding how functional changes occur.
Characterization of antibody structure is a key component in identifying such distinctions. Evaluating biomolecule stability at various thermal conditions is critical to ensuring these pharmaceuticals’ quality and regulatory approval.
Differential Scanning Calorimetry (DSC) is a thermal method for analyzing antibody structure and assessing a compound’s resistance to thermal stress.
Short-term thermal stability is an essential quality characteristic for assessing antibody pharmaceuticals. It is a fundamental metric utilized in candidate selection and pre-formulation. It is also a key factor for choosing buffer composition, such as excipients, buffer salts, detergents, and pH for developing clinical formulations.
It is common for tens to hundreds of specimens to be run and examined. Conventional DSC studies run slowly, testing one sample at a time, and require thorough cleaning between specimens for optimal outcomes.
The TA Instruments RS-DSC is a novel platform for high-throughput thermal stability testing that redefines thermal stability testing by concurrently analyzing 24 samples.
Swift and comprehensive physicochemical insights via thermal analysis reduce the time and cost of introducing novel drugs to market.
DSC thermograms for antibodies are remarkably varied. Some antibodies exhibit one peak, whereas others display several unique peaks, or intersecting peaks that appear as shoulders on a higher unfolding peak. Variation in these thermograms has displayed a correlation with the flexibility of the hinge region.1
Research on multi-domain proteins shows that these complex and overlapping peaks may arise from inter-domain interactions.2 Sophisticated DSC research has accurately identified the unfolding temperature of specific domains inside the heavy chain section of antibodies.3
The primary objective of such research is to determine thermal stability since inadequate stability can greatly affect solubility, resulting in aggregation—a frequent problem in biotherapeutics.

Figure 1. A) TA Instruments RS-DSC and B) disposable microfluidic chip (MFC) and cover slip. Image Credit: TA Instruments
Experiments and methods
Herceptin™ trastuzumab was manufactured under the package guidelines in histidine formulation buffer (18.4 mg/mL trehalose dihydrate, 0.08 mg/mL polysorbate 20, 0.49 mg/mL histidine HCl, 0.32 mg/mL histidine, pH 6.0) at 21 mg/mL and then kept at 4 °C before use. For study across various buffers, the antibody was buffer exchanged utilizing an Amicon™ Ultra 10kDa molecular weight cut-off centrifugal spin filter.
The borate buffer consisted of 50 mM boric acid, 50 mM NaCl, and 2 mM EDTA at pH 8.0. PBS, a typical working buffer, consisted of Gibco™ DPBS with 1 mM EDTA at pH 7.4. The succinate formulation buffer utilized for KADCYLA™, an antibody-drug conjugate developed on the trastuzumab antibody scaffold, consisted of 10 mM sodium succinate, 6% w/v sucrose, and pH 5.0.
Antibody specimens for mutation screening were developed and provided by Cell Signaling Technology and prepared in PBS (pH 7.4) at 20 mg/mL for examination.
The protein sample was pipetted straight into the disposable glass microfluidic chips (MFCs), which had a channel designed to hold 11 μL of liquid sample. Following the addition of the protein solution, the microfluidic chips were closed using adhesive-backed glass coverslips to hold the specimen while increasing the temperature to 100 °C.
The assembled MFCs were put onto the sample side of both twin calorimeters in the TA Instruments RS-DSC (Figure 1).
A reusable polyetheretherketone chip stays on the reference side. As many as 24 samples can be run simultaneously between temperatures of 20–100 °C alongside pre-set scan rates of 1 or 2 °C/min. By removing the requirement for cleaning between scans, as many as 96 samples may be analyzed in a standard day of work.
The TA Instruments RS-DSC, operated using RS-DSCRun software, was configured to equilibrate at the starting temperature for 1800 s before the initiation of each scan. All samples were analyzed in triplicate and scanned at 1 or 2 °C/min within the complete temperature range (20 to 100 °C).
For optimal precision across each of the 24 calorimeters, the device was initially adjusted and validated with dipalmitoylphosphatidylcholine, with an accepted Tmax shift of under 0.2 °C from the anticipated literature value.4
Data was processed using NanoAnalyze™ Software (v4.0.0). New functions in the Software enable automatic identification of the denaturation midpoint temperature (Tmax) and comparisons of up to 96 thermograms.
RapidDSC, a new software algorithm included in NanoAnalyze Software v4.0.0, automatically detects Tmax in DSC thermograms by looking for peaks throughout the given spectrum of information. An automatic baseline is applied across the peak and utilized to additionally refine the Tmax temperature for enhanced precision.
Automatic peak detection properties demonstrate as many as three peaks within the temperature range of interest. The automation characteristics may be effortlessly changed in the “Baseline and Tmax Editor” pop-out window to reduce the detection window, choose a defined range for peak identification, or change the automated baseline and Tmax.
Tabulated information from Tmax automation can be transferred for smooth comparison or can be viewed in RapidDSC’s Tmax visualization and overlay tabs.
Manual evaluation in NanoAnalyze Software was carried out to compare to the automated Tmax values that were identified via RapidDSC. Each individual scan was normalized to molar heat capacity utilizing the moles of protein in the active cell volume for manual comparison to the automated peak detection features. A fourth- or fifth-order polynomial was applied as the baseline of the normalized data.
For each transition, Tmax was visually predicted and then compared to the automated Tmax output. The thermograms may be further analyzed by fitting with models as outlined in “NanoDSC: What to Consider when Choosing a Baseline and Model”.5 It is recommended to use Voigt models to analyze antibody stability.
Results and discussion
Formulation buffer screening
Thermal stability is a crucial characteristic of any biologic pharmaceutical. DSC is the main instrument used to define the solution environment’s impact on protein stability. Protein stability is affected by various solution environment variables, including pH, buffer type, ionic strength, excipients, and detergents.
These impacts can be seen as small shifts in the Tmax or larger variations of up to tens of degrees.6 Developed with the demands of the biopharmaceutical industry in mind, the parallel throughput of the TA Instruments RS-DSC allows screening of solution conditions for biologic protein drugs, enhancing the time to decision over conventional microcalorimetric techniques.
To illustrate how the information from formulation screening can help with choosing buffer parts, TA Instruments examined triplicate data from the antibody Herceptin trastuzumab in four typical buffer environments (as shown in Figure 2 and Table 1).
For this research, antibodies in different formulation buffers were assessed at ~ 20 mg/mL; however, the TA Instruments RS-DSC has been specially developed for handling formulation strength protein concentrations of up to 330+ mg/mL, enabling thermal stability assessments of undiluted pharmaceuticals.
For additional details, check TA Instruments’ article “Rapid Thermal Stability Screening of High Concentration Biologic Drugs”.7
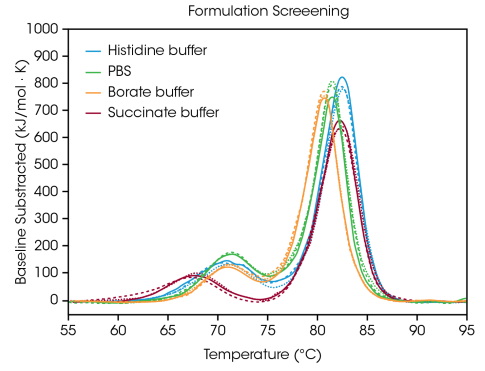
Figure 2. Overlay of replicate data from screening of Herceptin Trastuzumab in histidine, borate, PBS, and succinate buffer. Data collected in triplicate at an antibody concentration of ~20 mg/mL with a scan rate of 1 °C/min. Image Credit: TA Instruments
Table 1. Transition temperatures for formulation screen. Mean ± SD, n = 3. Source: TA Instruments
| Buffer |
Tmax,1 (°C) |
Tmax,2 (°C) |
| Histidine |
70.76 ± 0.38 |
82.66 ± 0.09 |
| Borate |
70.84 ± 0.08 |
80.69 ± 0.02 |
| PBS |
71.12 ± 0.06 |
81.48 ± 0.02 |
| Succinate |
67.71 ± 0.22 |
82.66 ± 0.08 |
The initial unfolding event, linked to the unfolding of the CH2 domain, is not greatly affected by the histidine, borate, or PBS buffers.8 The succinate buffer destabilizes the CH2 domain leading to a decrease in the onset of unfolding and Tmax,1 around 3 °C.
In terms of the primary transition reflecting the Fab and CH3 unfolding events, the histidine and succinate buffers are the most stabilizing, possessing a Tmax,2 of 82.66 °C. The primary transition is least stable in the borate buffer with a Tmax,2 of 80.69 °C. Naturally, the most stabilizing buffer formulation for trastuzumab in this sample set is the histidine buffer utilized for the finalized formulation of the approved medication.
To enable data analysis of formulation screening, automated Tmax detection in NanoAnalyze Software permits precise identification for as many as three transitions in protein stability screens. To demonstrate the precision of the peak-selecting algorithm, auto Tmax,1 and Tmax,2 for trastuzumab at 50 mg/mL in histidine formulation buffer are contrasted with manual peak selecting and shown in Figure 3 and Table 2.
For automatic examination, two peaks were identified following the application of a fourth-order baseline polynomial utilizing the auto startup hook detection function, which does not need to specify the temperature range of interest.
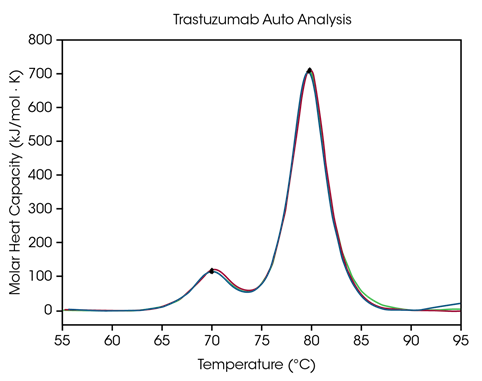
Figure 3. Overlay graph of triplicate scans generated with RapidDSC using automated Tmax and baseline detection of antibody thermograms in triplicate at 50 mg/mL. Detected Tmax values indicated with a black dot. Image Credit: TA Instruments
Table 2. Comparison between automated and manual Tmax detection for trastuzumab. Source: TA Instruments
| Sample |
Auto Tmax,1 (°C) |
Manual Tmax,1 (°C) |
Auto Tmax,2 (°C) |
Manual Tmax,2 (°C) |
| Replicate 1 |
71.93 |
72.04 |
82.92 |
82.88 |
| Replicate 2 |
71.81 |
71.93 |
82.76 |
82.75 |
| Replicate 3 |
71.93 |
71.97 |
82.90 |
82.83 |
| Average |
71.89 ± 0.07 |
71.98 ± 0.06 |
82.86 ± 0.09 |
82.82 ± 0.07 |
The CH2 domain unfolding possesses an autonomous Tmax,1 of 71.89 °C compared to 71.98 °C for manual peak analysis. The primary transition features an autonomous Tmax,2 of 82.86 °C compared to 82.82 °C for manual peak assessment. Ideal reproducibility for trastuzumab is observed.
Using both manual and automated peak analysis, the Tmax of the two peaks is shown to be inside the tool’s accuracy restrictions ± 0.2 °C, when tested in triplicate. A visual assessment is always advised to guarantee proper data comprehension. This data set shows that the automated DSC analysis properties offer precise detection of Tmax in multi-transition thermograms.
Screening of sequence modifications
Protein mutations are commonly used techniques for improving protein structure and function, and even just one amino acid modification could lead to a sizable effect on the general stability of proteins. DSC serves to appreciate the effects of a mutation on the thermal stability of a protein and may assist in guiding the decision-making across the biologic drug development pipeline.
To illustrate the various kinds of impacts a sequence modification may have on stability, a small panel of engineered antibodies was tested for variations in thermal unfolding stemming from a single amino acid mutation in the protein sequence (as shown in Figure 4 and Table 3).
Unfolding of the CH2, Fab, and CH3 domains are contained in one major thermal transition at a Tmax of 75.92 °C in the parent antibody. One amino acid mutation was generated from the parent protein with no significant impact on short-term thermal stability (Mutation 1) with a Tmax of 75.52 °C.
In contrast, further single amino acid mutations are illustrated to have major effects on protein stability (Mutation 2 and Mutation 3). The presence of a visible shoulder is noted in Mutation 2, likely corresponding to destabilization in the CH2 domain with an additional Tmax,1 of 70.04 °C accompanied by a slight shift in the principal transition relative to the parent protein with a Tmax,2 of 75.14 °C.
Mutation 3 results in major destabilization of the CH2 domain, leading to a complete additional unfolding peak with a Tmax,1 of 64.85 °C. The primary transition is largely unaffected with a Tmax,2 of 75.29 °C. As demonstrated in the major destabilization in Mutation 3, modification does not have the same effect every time.
Instead, it relies on the modification site and the physicochemical characteristics of the novel amino acid. Maximizing the intended operational advantages of sequence modification with the structural stability of the protein helps understand the structure-function relationship and may assist in developing sophisticated medicines.
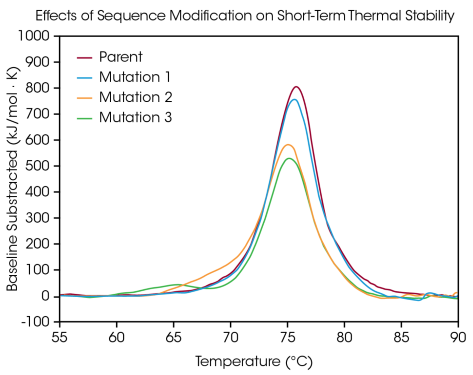
Figure 4. Modification of a single amino acid in the engineered antibody can impact overall short-term thermal stability. Data collected at ~20 mg/mL protein concentration with a scan rate of 1 °C/min Image Credit: TA Instruments
Table 3. Transition temperature for mutation screen. Mean ± SD, n = 3. *Determined via Voigt Model. Source: TA Instruments
| Sample |
Tmax,1 (°C) |
Tmax,2 (°C) |
| Parent |
— |
75.92 ± 0.15 |
| Mutation 1 |
— |
75.52 ± 0.14 |
| Mutation 2 |
70.04 ± 0.86* |
75.14 ± 0.06 |
| Mutation 3 |
64.85 ± 0.05* |
75.29 ± 0.06 |
Conclusions
Screening the effects of solution environment and sequence modification on short-term thermal stability is crucial for developing superior pharmaceutical drugs. The TA Instruments RS-DSC allows high-throughput screening for thermal stability via simultaneous testing of up to 24 samples at a time, substantially reducing time to decision.
In this study, TA Instruments shows precise and reproducible examination of complex-multiple transition antibody specimens, and the quick determination of buffer component and mutation effects on overall protein structure. Novel algorithms in the data processing software, NanoAnalyze, enable the study of huge amounts of thermal information presently accessible through high throughput screening.
In general, the TA Instruments RS-DSC offers a novel platform for assessing antibody drug product stability—a key factor for comprehending resiliency to thermal stress and product quality, as well as supporting regulatory approval.
References and further reading
- V. T. Oi, T. M. Vuong, R. Hardy, J. Reidler, J. Dangl, L. A. Herzenberg and L. Stryer, “Correlation between segmental flexibility and effector function of antibodies,” Nature, vol. 307, pp. 136-140, 1984.
- P. L. Privalov and S. A. Potekhin, “Scanning microcalorimetry in studying temperature-induced changes in proteins,” Methods in Enzymology, vol. 131, pp. 5-51, 1986.
- J. Wen, Y. Jiang and L. Nahri, “Effect of Carbohydrate on Thermal Stability of Antibodies,” American Pharmaceutical Review, vol. 11, p. 98, 2008.
- “Standard Practice for Calibration of Fixed-Cell Differential Scanning Calorimeters,” ASTM E2603-15, 2023.
- “NanoDSC: What to Consider when Choosing a Baseline and Model,” TA Instruments, MC154.
- C. M. Johnson, “Differential scanning calorimetry as a tool for protein folding and stability,” Archives of Biochemistry and Biophysics, vol. 531, pp. 100-109, 2013.
- “Rapid Thermal Stability Screening of High Concentration Biologic Drugs,” TA Instruments, MC177.
- K. J. Arlotta, A. V. Gandhi, H.-N. Chen, C. S. Nervig, J. F. Carpenter and S. C. Owen, “In-Depth Comparison of Lysine-Based Antibody-Drug Conjugates Prepared on Solid Support Versus in Solution,” Antibodies, vol. 7, p. 6, 2018.
About TA Instruments
TA Instruments designs and manufactures the world’s highest performing thermal analysis, rheometer, microcalorimeter, and mechanical test systems by focusing on four fundamental measurements: temperature, force, weight, and displacement.
No other company or combination of companies sells as many of these systems as TA Instruments. Our products are used on a daily basis in the world’s most prestigious companies, universities and government laboratories.
Headquartered in New Castle, Delaware, TA manufactures at 7 different locations in the United States and Europe, and has direct sales and support staff in 24 countries and 5 continents.
TA Instruments has long been recognized for the technical competence and professionalism of our sales force. We are consistently ranked #1 by third party surveys for our responsive and knowledgeable support, the hallmark of our company.
TA Instruments reputation for high performance products, quality manufacturing, and unbeatable after sales support is why more customers recommend TA products to their colleagues, compared to any other vendor. Here is what TA customers say about TA in a recent survey:
TA Instruments – Product Guide

Thermal Analyzers - Differential Scanning Calorimetry, Thermogravimetric Analyzers, Simultaneous Thermal Analyzers, Vapor Sorption Analyzers, Dynamic Mechanical Analyzers, Thermomechanical Analyzers

Rheometers - Discovery Hybrid Rheometers, ARES-G2 Rheometers, Dynamic Mechanical Analyzers, Rubber Rheometers

Microcalorimeters - Differential Scanning Calorimetry, Isothermal Titration Calorimetry, Isothermal Calorimetry

Dilatometers - Horizontal Dilatometers, High Resolution Horizontal Dilatometers, Vertical Dilatometers, Quenching Dilatometers, Optical Dilatometers, Heating Microscopes, Optical Platform

Thermal Conductivity Meters - Insulation & Building Materials, Heat Flow Meters, Guarded Hot Plate

Laser and Xenon Flash Analyzers

ElectroForce Mechanical Test Instruments - Load Frames, High Force Dynamic Mechanical Analyzers, TestBench and Planar Biaxial Testing, Cardiovascular Testing, Tissue Engineering

Rubber Testing Instruments - RPA, MDR, Mooney, Density & Hardness Testers, Sample Cutter, Mechanical Fatigue Testing/DMA

Primary Activity
Service Provider
Thermal analysis, rheometer and microcalorimetry supplier.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.