Dynamic Vapor Sorption (DVS) is a potent analytical technique used in the pharmaceutical industry to determine how much vapor a sample absorbs or desorbed under regulated temperature and humidity conditions.
This technique offers valuable insights into the behavior of pharmaceutical materials, affecting everything from stability and shelf life to formulation and manufacturing processes. In this article, Surface Measurement Systems will examine the many advantages of DVS throughout the pharmaceutical industry.

Image Credit: Surface Measurement Systems Ltd
Amorphous content determination
One notable advantage of DVS is its ability to detect low levels of amorphous content in pharmaceutical powders. Amorphous content can lead to instability and decreased efficacy, as these materials tend to absorb more moisture, resulting in phase transitions and product degradation.
DVS effectively quantifies amorphous content by analyzing moisture sorption isotherms. It enables scientists to calibrate known amorphous levels or identify vapors that trigger crystallization, providing insight into the extent of amorphous phases.
For instance, the data presented shows how the crystallization of a pharmaceutical ingredient was induced using ethanol as a solvent (see Figure 1).
After establishing a baseline, the system was set to a low partial pressure environment before initiating crystallization at 30 % p/p0. To induce a phase change, ethanol concentration was then increased to 85 % p/p0, leading to the formation of a crystalline material.
This structured crystalline lattice resulted in reduced uptake of ethanol when the system was returned to 30 % p/p0 during the third stage of the experiment. The amorphous content was determined by the difference in ethanol uptake between the first and third stages of the experiment.
Such studies help improve pharmaceutical stability and shelf life by identifying and managing unstable amorphous regions.
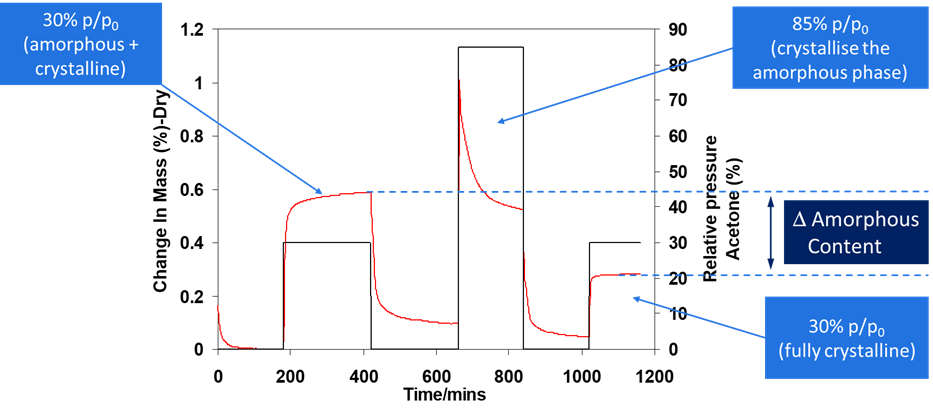
Figure 1. The determination of amorphous content of a pharmaceutical material using the DVS. Image Credit: Surface Measurement Systems Ltd
Analysis of drug excipient moisture interactions
Moisture sorption isotherms, which illustrate the equilibrium moisture content of a material across various relative humidity levels, are crucial for understanding and predicting the behavior of pharmaceuticals under different environmental conditions.
DVS enables precise measurement of these isotherms, offering detailed insights into the hygroscopic properties of both active pharmaceutical ingredients (APIs) and excipients. This information is vital for designing stable and effective formulations.
For example, analyzing the moisture sorption characteristics of hydrophobic pharmaceutical substances can be revealing.
Figure 2 shows the water sorption isotherms for hydroxypropyl cellulose (HPC), microcrystalline cellulose (MCC), and a Lovastatin mixture. The percent change in mass (relative to the dry mass) is plotted on the y-axis, while the target relative humidity is plotted on the x-axis.
Lovastatin, being highly hydrophobic, shows minimal mass change between 0 and 95 % RH. In contrast, HPC and MCC, which are significantly more hydrophilic, exhibit more pronounced moisture absorption.
DVS helps elucidate how these materials interact with moisture, aiding in the selection of appropriate excipients and optimization of formulations to enhance drug performance and extend shelf life.
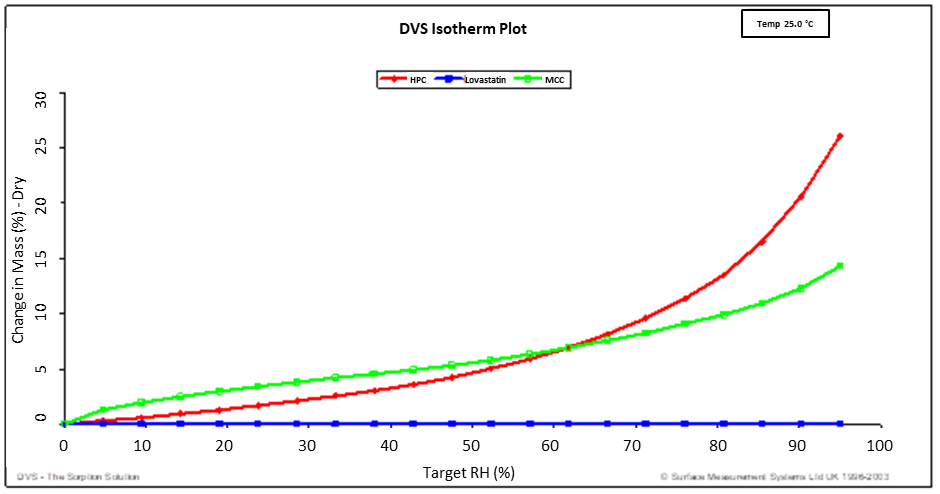
Figure 2. Water sorption isotherms for HPC (red), MCC (green), and Lovastatin (blue) 25.0 °C. Image Credit: Surface Measurement Systems Ltd
Determination of glass transition RH
The DVS can determine the crucial relative humidity (RH) at which a glass transition takes place. This is crucial because the glass transition can cause major changes in a material's physical properties. Figure 3 is a DVS ramping experiment combined with a camera to analyze spray-dried lactose.
The experiment begins with a stable baseline and then gradually increases the relative humidity by 10 % per hour until reaching 90 %. Initially, moisture adsorbs on the surface of the material. As the relative humidity rises, lactose undergoes a glass transition, causing moisture to penetrate the bulk of the material. This increased moisture uptake enhances molecular mobility, leading to recrystallization once the relative humidity exceeds 80 %.
This absorbed moisture serves as a plasticizer, causing lactose molecules to reorganize and produce a solid crystalline structure. Further increases in RH cause material breakdown.
Identifying the glass transition RH allows manufacturers to better understand and control the storage and processing conditions of amorphous medicines.
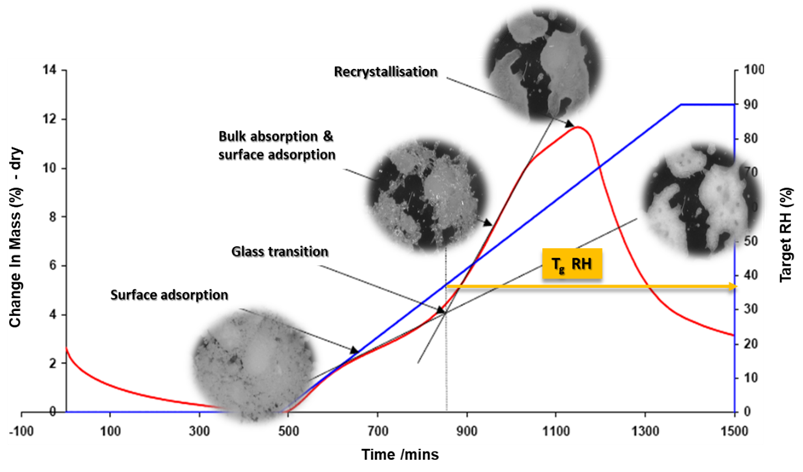
Figure 3. Ramping experiment of spray-dried lactose from 0-90% RH at a rate of 10% RH per hour showing a phase change through the glass transition stage with sample photos at each stage. Image Credit: Surface Measurement Systems Ltd
Packaging materials
Packaging materials protect food products from external conditions, and their capacity to interact with moisture has a substantial impact on shelf life and quality.
The DVS can be used to investigate how packing materials absorb and desorb moisture under different humidity conditions, providing information on their water-vapor transfer rates and barrier qualities.
The DVS can measure moisture diffusion coefficients by exposing package samples to controlled humidity conditions and detecting weight changes over time. This information is critical for selecting proper packing materials that keep the food stable and prevent spoiling, caking, or flavor and fragrance loss.
In addition, DVS can detect crucial humidity levels that may cause phase transitions or deterioration in packaging materials, allowing producers to create more effective packaging solutions that extend shelf life and improve food product preservation.
Improvements in quality control and assurance
DVS is a significant instrument for quality control in pharmaceutical manufacturing. It ensures that materials meet the moisture content parameters required to maintain product quality and regulatory compliance. Regular usage of DVS in quality assurance processes ensures that each batch of goods meets the desired criteria.
Dynamic Vapor Sorption (DVS) is a critical technology in the pharmaceutical business, providing increased sensitivity, thorough moisture sorption research, and important insights into drug-excipient interactions.
Its capacity to identify tiny amounts of amorphous material, investigate complex formulations, and verify product stability and quality make it an essential tool for pharmaceutical research, development, and manufacture.
In the research and development phase, the DVS speeds up the formulation process by delivering quick and reliable data on the moisture-related properties of new compounds and formulations.
This allows researchers to make more informed judgments early in the development process, decreasing the time and expense associated with bringing novel medications to market.
As the industry evolves, DVS will play an increasingly important role in guaranteeing the efficacy and safety of pharmaceutical goods, encouraging innovation, and improving patient outcomes.
About Surface Measurement Systems Ltd
 Surface Measurement Systems Ltd develops and engineers innovative experimental techniques and instrumentation for physico-chemical characterisation of complex solids. We are the world leaders in Dynamic Vapor Sorption technology and Inverse Gas Chromatography instrumentation and solutions, providing professional world-class scientific and technical support for our international customers. By carefully controlling, measuring and analysing the physico-chemical interaction of vapors with solid samples such as powders, fibres and films, Surface Measurement Systems Ltd can help solve problems in research and development, such as stability studies and drying performance, through to manufacturing and quality control.
Surface Measurement Systems Ltd develops and engineers innovative experimental techniques and instrumentation for physico-chemical characterisation of complex solids. We are the world leaders in Dynamic Vapor Sorption technology and Inverse Gas Chromatography instrumentation and solutions, providing professional world-class scientific and technical support for our international customers. By carefully controlling, measuring and analysing the physico-chemical interaction of vapors with solid samples such as powders, fibres and films, Surface Measurement Systems Ltd can help solve problems in research and development, such as stability studies and drying performance, through to manufacturing and quality control.
Our range of characterization instruments and scientific/engineering techniques has helped solve difficult problems in the pharmaceuticals, biomaterials, polymers catalysts, chemical, cosmetics and food industries, and are used by hundreds of leading laboratories and universities throughout the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.