Selected ion flow tube mass spectrometry (SIFT-MS) greatly simplifies quantitative ethylene oxide (EtO) analysis in Polysorbate 80 (P80) excipient. It has a time-to-first test result eight times faster than the existing compendial method and a daily sample throughput nine to 14 times higher.
Image Credit: ShutterStock/Irina Anosova
EtO is broadly utilized as a feedstock in chemical production, including in producing polyethylene glycols (PEGs), used as emulsifiers and surfactants in perfumes, cosmetics, and pharmaceuticals.1
EtO is also commonly used as a sterilant in pharmaceuticals and medical implant engineering owing to its sporicidal, bactericidal, and virucidal properties. It is also compatible with numerous biomaterials with which heat sterilization and radiation are otherwise incompatible.2
Such toxicity is not limited to microbes: it presents a mutagenic risk to humans, making it vital to detect trace EtO impurities.3
P80, a poly EtO existing under various trade names, including Tween 80® (T80), is generally used as an emulsifier in many pharmaceutical products.4 The United States Pharmacopeia monograph on poly EtO summarizes the classical gas chromatography-flame ionization detection (GC-FID) technique for EtO investigation.5
This methodology is laborious from the viewpoints of sample preparation and testing.6 The rate-limiting stage in sample preparation is the six-hour purification of the polyethylene glycol (PEG) matrix corresponding to the sample (1 mL of P80 in 1 mL of N, N-dimethylacetamide, and 0.2 mL of water). The GC-FID cycle runtime is 38 minutes per sample.
This article discusses an efficient alternate analytical technique. By utilizing a more sensitive headspace method combined with SIFT-MS, a considerably smaller amount of P80 is needed than that of headspace GC-FID.
This saving removes the need for matrix-matching and eliminates the six-hour PEG purification step.
Headspace SIFT-MS (hSIFT-MS) evaluation of EtO in P80 provides a nine-times larger sample throughput than GC-FID and achieves first test results quicker when blanks, calibrations, and a system suitability test are taken into account (85 minutes compared to six hours; Figure 1).
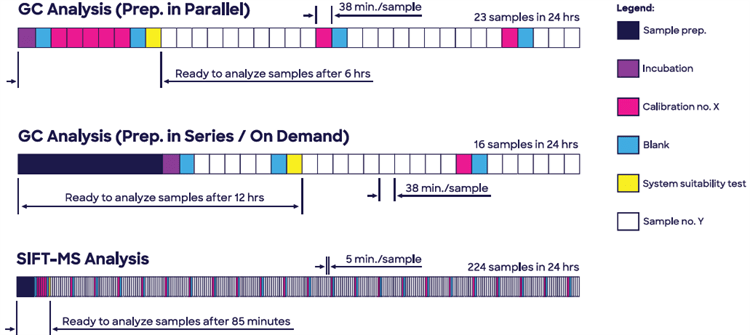
Figure 1. Headspace SIFT-MS enables analysis of ethylene oxide in Polysorbate 80 (Tween 80®) to be conducted at significantly higher throughput while eliminating very slow sample preparation due to enhanced sensitivity. Image Credit: Syft Technologies
SIFT-MS Procedure
A Syft Technologies Voice200ultra SIFT-MS device working on helium carrier gas was used for this experiment. SIFT-MS (Figure 2) uses soft chemical ionization to yield mass-selected reagent ions, which can react quickly with and quantify volatile organic compounds (VOCs) close to part-per-trillion concentrations.7
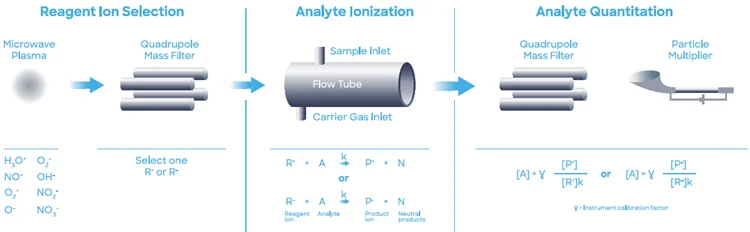
Figure 2. Schematic diagram of SIFT-MS – a direct, chemical-ionization analytical technique. Image Credit: Syft Technologies
A maximum of eight reagent ions (H3O+, NO+, O2+, O-, OH-, O2-, NO2-, and NO3-) attained from microwave discharge in the air are currently used in commercial SIFT-MS devices.8
These reagent ions react to VOCs and other trace analytes in well-controlled ion-molecule reactions but do not respond to the primary components of air (N2, O2, and Ar).
This allows for direct evaluation of air samples in real-time at trace and ultra-trace levels without pre-concentration. Fast switching between reagent ions gives better selectivity, and the various reaction mechanisms offer measurements of each analyte.
The numerous reagent ions constantly remove uncertainty from isobaric overlaps in mixtures of multiple analytes.
Automated MHE evaluation was carried out utilizing a SIFT-MS instrument combined with a multi-purpose auto-sampler (MPS Robotic Pro, GERSTEL; Mülheim, Germany). GERSTEL’s Maestro software was utilized to operate the auto-sampler. Samples were incubated for 45 minutes at 80 °C in a GERSTEL agitator.
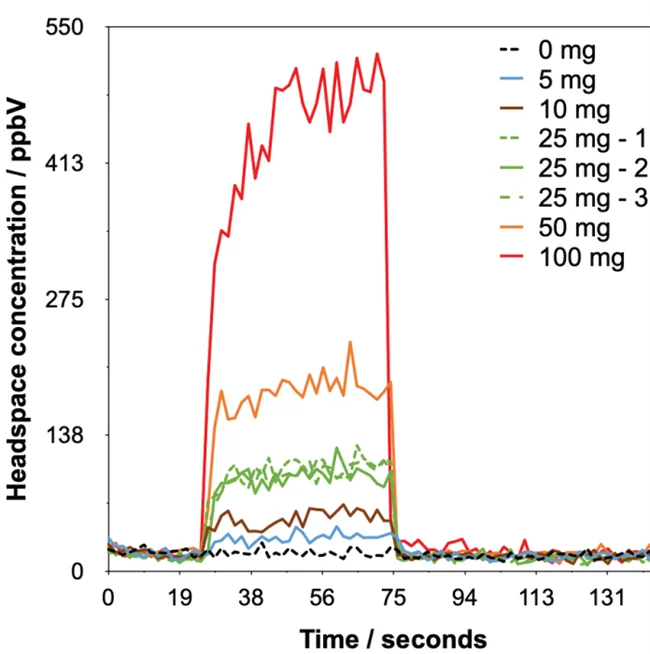
Figure 3. Example headspace injections with synchronous SIFT-MS analysis of ethylene oxide detected from Tween 80® at various dilution levels in water. Image Credit: Syft Technologies
Headspace was tested using a 2.5 mL headspace syringe heated to 150 °C; subsequently, it was injected at a flow rate of 50 μL s-1 into the SIFT-MS instrument’s auto-sampler inlet (also at 150 °C) via a GERSTEL self-sealing septumless sampling head.
As the nominal sample flow into the SIFT-MS instrument is 420 µL s-1, a make-up gas flow (ultra-high purity nitrogen) was also introduced through the sampling head.
The calibration procedure automatically accounts for this dilution. Each sample took 145 seconds to analyze (Figure 3), and the concentrations stated are the mean of the values measured during injection (ca. 40 to 70 seconds). No internal standard was used.9
SIFT-MS freely identifies EtO (Table 1). Reaction rate coefficients (k) are the main measure of SIFT-MS sensitivity.
The NO+ reagent ion is significantly less sensitive to EtO than H3O+ and O2+ reagent ions. The NO+ product ion (m/z 74) can only be used at higher concentrations of EtO.
Linear measurement in the gas phase is shown in Figure 4. Acetaldehyde is the main potential interferer for EtO, similar to the compendium GC-FID method, though both require very different analytical approaches.10
A summary of the SIFT-MS reaction chemistry of acetaldehyde is shown in Table 1.
Table 1. SIFT-MS reaction chemistry (rate coefficient (k), product ion formulae, branching ratio (BR as %), and mass-to-charge ratio (m/z)) for ethylene oxide (EtO) and acetaldehyde, a potential interferent. Ions in gray are not ordinarily used for quantitation because they are frequently occurring product ion m/z for other VOCs. Source: Syft Technologies
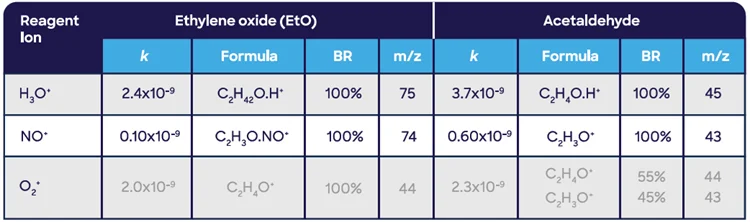
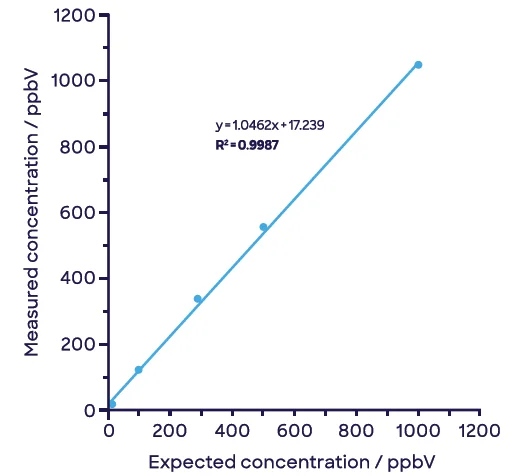
Figure 4. Linear measurement of ethylene oxide in the gas phase using SIFT-MS. Image Credit: Syft Technologies
T80 samples often have low EtO levels, and this work is intended to leverage the maximum sensitivity to limit sample size and alleviate matrix effects, so a subtraction approach was applied.
The H3O+ reagent ion was used to assess the combined concentration of EtO and acetaldehyde. NO+ was utilized to calculate the acetaldehyde concentration, permitting the determination of EtO via the subtraction of the latter from the former value.
Figure 5 shows how these compounds can be separated simply via this approach. The insignificant signal of acetaldehyde remains so with growing concentrations of EtO (Figure 5[a]); the reverse is shown with increasing concentrations of acetaldehyde (Figure 5[b]).
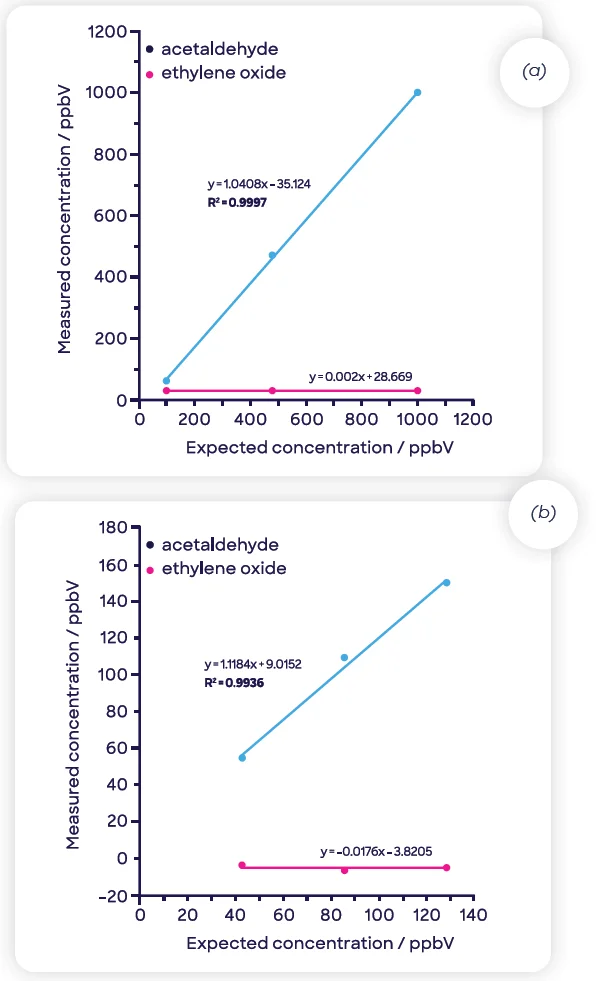
Figure 5. Effective discrimination and linear detection of (a) ethylene oxide and (b) acetaldehyde in samples containing both compounds. Image Credit: Syft Technologies
Samples
T80 was the commercial P80 product used in this study (Croda, Inc., Edison, New Jersey).
Compared to the compendium method, which uses 1 g of sample per test, the quantity of T80 used in this study ranged from 5 to 100 mg, with water balance supplying a total sample volume of 1 mL.11
The choice was made to reduce the sample volume to 1 mL instead of the standard USP sample volume of 2.2 mL, as incubation times and headspace equilibration had been optimized to accommodate the lower volume.
Results and Discussion
Small quantities of T80 in water were analyzed to find the limit of quantitation for the SIFT-MS evaluation of EtO partitioning from T80 and water mixtures.
Figure 3 shows the SIFT-MS results, which indicate that EtO can be perceived above baseline even when just 5 mg of T80 is used (signal-to-noise ratio: 5:0).
Triplicate samples were evaluated at 25 mg, offering a relative standard deviation of 4.5 %, which is in line with other hSIFT-MS studies.12
Figure 6(a) shows the EtO response attained using hSIFT-MS as the load of T80 was altered (between 5 and 94 mg). Because the matrix composition fluctuates as the amount of T80 increases, the quadratic fit shows that there may be a matrix effect even at lower dilution levels (50 and 100 mg).
Figure 6(b) displays a linear fit (regression coefficient (R2) of 0.996) acquired at higher dilutions (between 5 and 25 mg of T80), showing that in this range, the matrix effects are removed and that the system has a consistency similar to that of water.
Such high dilution levels are well-suited to SIFT-MS but not GC-FID because of the former technique’s greater sensitivity.
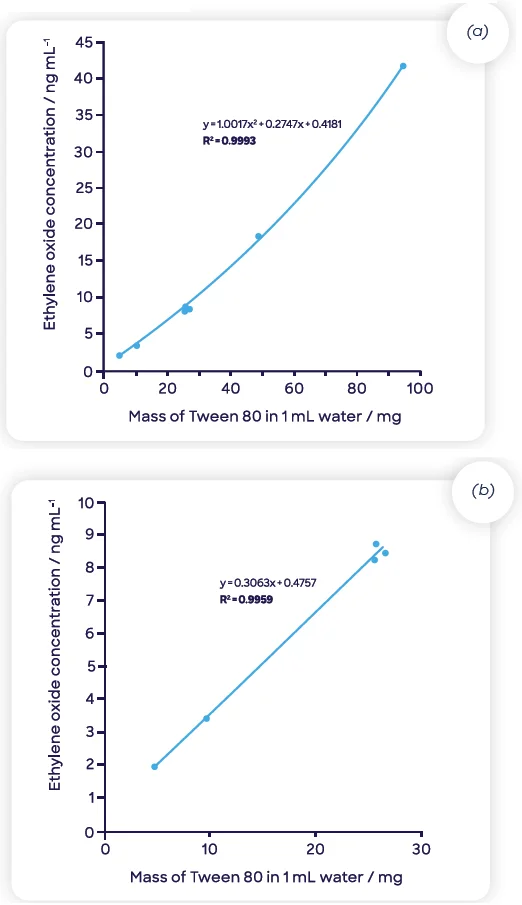
Figure 6. Ethylene oxide concentrations for Tween 80® diluted in water determined using headspace-SIFT-MS analysis (a) across the full dilution range and (b) at the three highest dilution levels (where matrix effects are eliminated). Image Credit: Syft Technologies
Figure 7 shows the mean of triplicate EtO measurements of the 25 mg T80 sample in water and the aqueous 100 ng mL-1 EtO standard. For the single-point calibration in water, 8.47 ng of EtO was obtained for the 25 mg T80 sample, yielding a concentration in the T80 sample of 339 ng g-1 or 0.339 ppm (w/w).
Matrix effects did not need to be adjusted; they were insignificant at these comparatively high dilution levels.
It can be presumed that when conducting tests on EtO in P80 with hSIFT-MS, a sample of only 10 mg needs to be introduced to 1 mL of water, with calibration performed against an external standard prepared in water.
This eradicates the requirement to purify PEG for matrix-matching the calibration standard when using GC-FID and significantly reduces the sample preparation for experimentation.
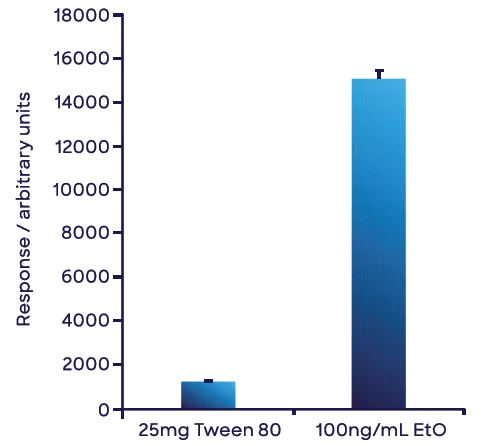
Figure 7. Measurement of the concentration of ethylene oxide in Tween 80® based on a single-point external calibration. Both solutions were prepared in water. The small volume of Tween 80® used negated any matrix effects from PEG. The error bars represent 1 standard deviation of triplicate measurements. Image Credit: Syft Technologies
Conclusions
A highly sensitive hSIFT-MS evaluation can perceive EtO in a sample mass 100 times lower than that used in classical methods, facilitating the elimination of the six-hour preparation period required for matrix-matched standards.
hSIFT-MS testing of EtO is nine to 14 times quicker than the compendium GC-FID method and can generate sample throughputs of up to 224 samples a day.
By removing matrix-matched standards and delivering rapid analysis, SIFT-MS can give the first test result eight times quicker than GC-FID. Easy sample preparation and use and industry-proven technology make SIFT-MS well-suited for QA/QC labs and process lines.
References and Further Reading
- Wikipedia (2020) Ethylene oxide. Available at: https://en.wikipedia.org/wiki/Ethylene_oxide. (Accessed: September 20, 2022.)
- Qiu, Q. -q., Sun, W. -q. and Connor, J. (2017). 4.12 Sterilization of Biomaterials of Synthetic and Biological Origin ☆. Elsevier eBooks, pp.180–199. https://doi.org/10.1016/b978-0-12-803581-8.10186-9.
- Aronson, J. K. (2016) EtO. Meyler’s Side Effects of Drugs, 16th edn., Elsevier, pp.198-202. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780444537171007137.
- Wikipedia 2. Polysorbate 80. Available at: https://en.wikipedia.org.
- USP (2015). Polysorbate 80. Available at: https://www.uspnf.com/notices/polysorbate-80. (Accessed: September 20, 2022.)
- USP (2013). 〈228〉EtO and Dioxane.
- Smith, D., McEwan, M.J. and Španěl, P. (2020). Understanding Gas Phase Ion Chemistry Is the Key to Reliable Selected Ion Flow Tube-Mass Spectrometry Analyses. Analytical Chemistry, 92(19), pp.12750–12762. https://doi.org/10.1021/acs.analchem.0c03050.
- Hera, D., Langford, V., McEwan, M., McKellar, T. and Milligan, D. (2017). Negative Reagent Ions for Real Time Detection Using SIFT-MS. Environments, 4(1), p.16. https://doi.org/10.3390/environments4010016.
- Perkins, M. and Langford, V.S. (2021). Application of Routine Analysis Procedures to a Direct Mass Spectrometry Technique: Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Reviews in separation sciences, 3(1), pp.e21003–e21003. https://doi.org/10.17145/rss.21.003.
- Perkins, M. and Langford, V.S. (2022). Multiple Headspace Extraction-Selected Ion Flow Tube Mass Spectrometry (MHE-SIFT-MS). Part 1: A Protocol for Method Development and Transfer to Routine Analysis. Reviews in separation sciences, 4(1), pp.e22001–e22001. https://doi.org/10.17145/rss.22.001.
- Perkins, M.J. and Langford, V.S. (2021). Standard Validation Protocol for Selected Ion Flow Tube Mass Spectrometry Methods Applied to Direct Headspace Analysis of Aqueous Volatile Organic Compounds. Analytical Chemistry, 93(24), pp.8386–8392. https://doi.org/10.1021/acs.analchem.1c01310.
- EMA (2001) Note for guidance on limitations to the use of EtO in the manufacture of medicinal products. Available at: https://www.ema.europa.eu/en. (Accessed: September 20, 2022.)
About Syft Technologies
Syft Technologies is the world leader in real-time, direct injection mass spectrometry with more than 20 years of SIFT-MS expertise. The unique attributes of Selected Ion Flow Tube Mass Spectrometry (SIFT-MS) have enabled our customers to remove bottlenecks and solve daunting analytical challenges. The results are improved capacity and organizational efficiency for our customers and a safer world.
Syft Technologies supports a broad range of worldwide industries including semiconductor manufacturing, pharma and CDMOs, environmental protection, automotive, food, flavor and fragrance, and many more. Syft has offices throughout the world offering 24/7 service and support including those in New Zealand, Korea, Taiwan, Singapore, Germany and the U.S.
SIFT-MS delivers real-time, chromatography-free direct analysis of compounds that traditionally require intensive sample preparation. No sample preparation is required for even complex matrices and high-humidity samples. SIFT-MS analyzes compounds that cannot be easily targeted by traditional chromatographic methods such as formaldehyde, hydrogen sulfide, ammonia, ethylene oxide, and nitrosamines. SIFT-MS is industry-proven, providing non-technical operators with the laboratory-grade chemical analysis presented in a format that they can easily understand and act on. Syft’s flagship product is the Syft Tracer which delivers routine and repeatable analysis of volatile compounds.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.