Adeno-associated viruses (AAV) are powerful instruments in gene therapy. AAV particles comprise a protein capsid that along with the desired genome, containing both is considered a full capsid. However, the synthesization process is not guaranteed to always generate full capsids. It may produce partial or empty capsids that do not contain a genome or carry undesired contaminant DNA.
Analytical ultracentrifugation (AUC) is widely utilized to examine capsid content purity. However, it is unable to distinguish between desired genomes and contaminant DNA. This article contrasts findings from AAV empty-full capsid testing utilizing Droplet Digital™ PCR (ddPCR) and AUC. The Vericheck ddPCR Empty-Full Capsid Kit (Bio-Rad Laboratories, Inc., catalog #17010082 and #17010072) specially determines the therapeutic genome contents of AAV, delivering an analysis with higher degrees of precision analysis.
Introduction
The Vericheck ddPCR Empty-Full Capsid Kit may be utilized to determine AAV vectors that generate full, partial, and empty capsids. Although AUC is a useful approach for separating AAVs that contain different payloads, it cannot distinguish between capsids that contain the desired payload and contaminant DNA.
This is crucial, as contaminants can negatively affect health. This research examined capsids from two AAV serotypes via the AUC method and the Vericheck ddPCR Empty-Full Capsid Kit. The desired contents were determined using an AAV serotype 2 inverted terminal repeat (ITR-2) assay, and contaminants were determined via supplemental specific assays.
Material and methods
A commercial vendor received two AAV samples, one serotype 5 (AAV5) and one serotype 9 (AAV9). The two samples were generated via the triple plasmid transfection approach. The source vector maps are displayed in Figure 1.
Three tests were carried out with each AAV serotype utilizing the Vericheck ddPCR Empty-Full Capsid Kit according to its serotype and the ITR-2 assay, as discussed in the kit user guide.
The plate was examined via the QX600™ AutoDG™ ddPCR System (Bio-Rad, #17008371). The data were transferred from the QX Manager Software, Premium Edition, version 2.2 (Bio-Rad, #12018108). They were assessed utilizing the Vericheck ddPCR Empty-Full Capsid Analysis Worksheet, which produces genome and capsid titers outputs.
Each sample was diluted to around 1011 genome copies (GC)/ml and examined utilizing the Vericheck ddPCR Empty-Full Capsid Kit to quantify the percentage of capsids filled with ITR-2.
Each of the samples was studied following the manufacturer’s guidelines. In the case of non-ITR-2 targets, the default ITR-2 assay was substituted with the associated contaminant assay (Table 1). In addition, the KanR assay was examined together with the ITR-2 assay to quantify whether capsids contained the two targets. Each assay–sample combination was examined using three antibody binding reaction replicates and three ddPCR wells for each antibody binding reaction.
Table 1. Residual plasmid DNA assays. Source: Bio-Rad Laboratories
| Assay Target |
Product Name |
Description |
Bio-Rad Catalog # |
| KanR |
ddPCR Expert Design
Assay: KanR (HEX) |
Residual
GOI plasmid |
dEXD51863961 |
| Rep |
ddPCR RCAAV Assay |
Residual
Rep/Cap plasmid |
16011505 |
| E2A |
ddPCR Copy Number
Assay: dCNS919674709 |
Residual
pHelper |
dCNS919674709 |
GOI, gene of interest; RCAAV, replication competent adeno-associated virus.
Experimental analysis
All of the wells from the ddPCR assay were assessed utilizing QX Manager Software, Premium Edition. For each channel, the threshold was manually placed at a quarter of the distance between the center of the negative cluster and the center of the positive cluster, as demonstrated in Figure 2.
To quantify the percentage of full capsids in the ITR-2 assays and the residual plasmid targets, the output was transferred and examined using the Vericheck ddPCR Empty-Full Capsid Analysis Worksheet, as detailed in the kit user guide.
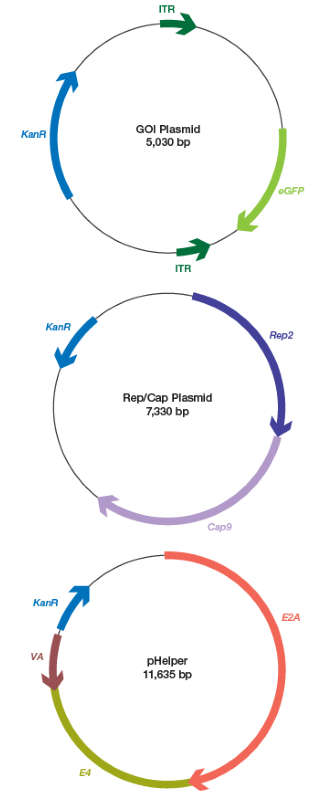
Fig 1. Vector genome maps for plasmids used in the AAV triple plasmid transfection assay. AAV, adeno-associated viruses; eGFP, enhanced green fluorescent protein; GOI, gene of interest; ITR, inverted terminal repeat. Image Credit: Bio-Rad Laboratories
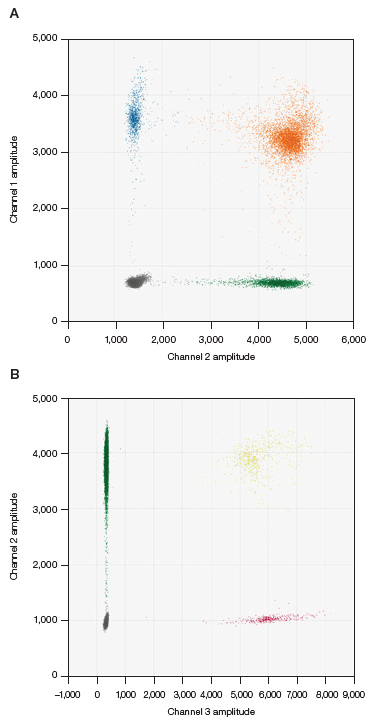
Fig. 2. Example of thresholding. A, capsid detection assay in FAM (●), ITR-2 assay in HEX (●), and double-positive clusters (●). B, KanR assay in FAM (●), ITR-2 assay in HEX (●), and double-positive clusters (●). Image Credit: Bio-Rad Laboratories
As the concentrations of the Rep and E2A assays were significantly lower than the concentration output of the capsid detection assay, it was impossible to analyze these targets and the capsid detection assay in the same well.
The percentage of capsids carrying Rep or E2A was quantitated by dividing the assessed concentration of Rep by the capsid titer quantified in the ITR-2 assay. The percentage of capsids carrying the plasmid backbone target and ITR-2 was quantitated using the QX Manager Software linkage calculation.
Results
Table 2 displays the proportion of full capsids for all of the approaches. Although the percentage of capsids filled with ITR-2 was smaller than that of filled capsids identified by AUC, ddPCR analysis utilizing the QX600 ddPCR System identified considerable quantities of contaminant fragments in the two samples.
Table 2. Percentage of full capsids calculated by AUC and ddPCR analysis. Source: Bio-Rad Laboratories
| |
|
Percent of capsids filled with ITR-2 and
contaminant fragment DNA* |
| Sample |
AUC, percent full capsids |
ITR-2 |
KanR |
E2A |
Rep |
| AAV5 |
69.8% |
54.8% |
4.9% |
0.1% |
0.14% |
| AAV9 |
81.8% |
71.8% |
2.6% |
0.1% |
0.11% |
AUC, analytical ultracentrifugation.
*Determined by ddPCR analysis using the QX600 System.
Continued analysis distinguished the proportion of capsids carrying the ITR-2 and a contaminating fragment, as displayed in Figure 3. For the two samples, most of the capsids carrying contaminant fragments did not simultaneously carry ITR-2.
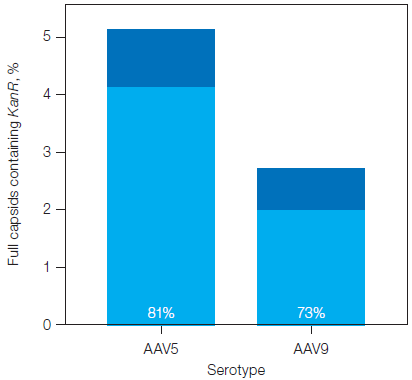
Fig. 3. Percentage of filled capsids in the plasmid backbone assays containing KanR, with and without ITR-2. The proportion of filled capsids containing a contaminant fragment but not containing ITR-2 is shown in light blue and represented by the percentage listed at the base of the bar. Image Credit: Bio-Rad Laboratories
Conclusions and discussion
The proportion of full capsids carrying ITR-2 was 10–15% below the total percentage of full capsids identified by AUC.
Yet, contaminant fragments from the plasmid backbone and up to 5% of full capsids were present in the two samples, which partly explains the discrepancy. Linkage analysis of the plasmid backbone and ITR-2 assays found that around 70–80% of capsids carrying the tested plasmid backbone assays did not also contain ITR-2.
These capsids would not be regarded as ‘full’ by the Vericheck ddPCR Empty-Full Capsid Kit but might be considered full by content-blind approaches, including AUC. It is plausible that additional capsids are filled with contaminant fragments from diverse sources, including residual host cell DNA, which would further exaggerate the number of full capsids identified by AUC.1,2,3
Only capsids that contain the desired vector genome produce the intended therapeutic effect. For biophysical approaches like AUC, all DNA is considered to be equivalent. Contrasting this, the Vericheck ddPCR Empty-Full Capsid Kit permits the measurement of capsids filled only with the desired vector genome, which correlate more strongly with product quality.
References and further reading
- Lecomte, E., et al. (2015). Advanced Characterization of DNA Molecules in rAAV Vector Preparations by Single-stranded Virus Next-generation Sequencing. Molecular Therapy - Nucleic Acids, 4, p.e260. https://doi.org/10.1038/mtna.2015.32.
- Aisleen McColl-Carboni, Dollive, S., et al. (2024). Analytical characterization of full, intermediate, and empty AAV capsids. Gene therapy. https://doi.org/10.1038/s41434-024-00444-2.
- Schnödt, M., et al. (2016). DNA minicircle technology improves purity of adeno-associated viral vector preparations. Molecular Therapy — Nucleic Acids, 5, e355. https://doi.org/10.1038/mtna.2016.60.
About Bio-Rad Laboratories
For over six decades, Bio-Rad has provided the healthcare industry with innovative and useful products that help life science researchers accelerate the discovery process and medical diagnostic labs obtain faster, better results.
Bio-Rad is among the top five life science companies in the world, providing instruments, software, consumables, reagents, and content for the areas of cell biology, gene expression, protein purification, protein quantitation, drug discovery and manufacture, food safety and environmental quality testing, along with science education. Our products and solutions are based on technologies to separate, purify, identify, analyze, and amplify biological materials such as antibodies, proteins, nucleic acids, cells, and bacteria.
As a leading global provider of in-vitro diagnostics supplies, our diagnostic products and systems leverage a broad range of technologies and deliver high-value clinical information in the blood transfusion, diabetes monitoring, autoimmune, and infectious disease testing markets. These products are used to support the diagnosis, monitoring, and treatment of diseases and other medical conditions.
Bio-Rad is the world leader in clinical quality control products, services, and information systems, products that ensure the accuracy and validity of clinical test results.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.