Quantification of empty and full adeno-associated virus (AAV) capsids is crucial for maximizing viral vectors used in gene therapy. The launch of the Vericheck ddPCR Empty-Full Capsid Kit expands this research by assessing the percentage of AAV capsids filled across multiple sample types with high precision, providing higher workflow flexibility and a more detailed assessment of AAV viral titer and capsid integrity.
Utilizing Droplet Digital™ PCR (ddPCR), Bio-Rad Laboratories developed a new technique for characterizing the percentage of filled capsids for different AAV serotypes. The kit provides a durable and dependable approach for examining capsid integrity, ensuring the consistency and efficacy of AAV-based therapies.
Introduction
AAVs are commonly utilized viral vectors in gene therapy and consist of a protein capsid alongside a recombinant DNA genome. Throughout synthesis, some AAV particles become mispackaged and lack some or all expected genes.
The Vericheck ddPCR Empty-Full Capsid Kit contains two assays - one assay dependant on the AAV serotype and the second assay that targets viral packaging sequences from AAV serotype 2 inverted terminal repeat (ITR-2).
Particles that contain ITR-2 are recorded as full capsids, while those without are recorded as empty. Yet, as shown here, different reference targets can also be utilized to produce a percentage of capsids with other targets - such as the recombinant DNA genome. If the percentage of filled capsids is not equal among different targets, this suggests the presence of capsids that have only been partly filled, providing a more comprehensive examination of genome integrity throughout AAV development.
Materials and methods
Summary
The steps that are detailed below may be utilized to expand the Vericheck ddPCR Empty-Full Capsid Kits (Bio-Rad Laboratories, Inc., catalog #17010072, #17010082) to other targets:
- Acquisition of ddPCR assay(s) for AAV targets in HEX.
- Replace the included ITR-2 assay with the desired assay(s). No alterations to the assay protocol or empty-full calculations are needed. However, annual thresholding of ddPCR wells may be needed.
- Contrasting the percentage of full capsids among targets to examine genome integrity.
Assay selection
The Vericheck ddPCR Empty-Full Capsid Kits comprise a capsid detection assay in FAM and an ITR-2 assay in HEX. The ITR-2 assay can be replaced with a different HEX assay to examine different genes packaged inside AAV capsid.
Researchers may ask for custom assays that utilize different predesigned vector backbones via the Bio-Rad Cell and Gene Therapy Assay Design Engine. Users can also design assays using the Droplet Digital PCR Applications Guide (bulletin 6407). Primers must be developed to have a melting temperature (Tm) of around 55 °C, with the Tm of the HEX probe being 3–10 °C higher than that of the primers. Every primer should be utilized at a 900 nM final concentration while probes should be used at 250 nM.
In the current research, Bio-Rad Laboratories examined AAV samples comprising ITR-2, the cytomegalovirus (CMV) promoter, and enhanced GFP (eGFP) for the presence of partial genomes. HEX-labeled assays against ITR-2 (Bio-Rad, dEXD23004642), the CMV promoter (Bio-Rad, dEXD96423937), and eGFP (Bio-Rad, dCGTS863952655) were taken from the Bio-Rad Cell and Gene Therapy Assay Design Engine.
Sample preparation
Three AAV serotype 9 (AAV9) samples carrying the genome ITR-2-CMV-eGFP-SV40-ITR-2 were utilized: Sample 1 (Charles River Laboratories, #CV10009), Sample 3 (Porton Advanced, #AAV9- DS230831-01), and Sample 4 (Charles River, #RS-AAV9-FL).
Sample 2 (Charles River, #RS-AAV9-ET) contained empty capsids and was diluted to around the same capsid concentration as Sample 1 with a dilution buffer by combining the same volumes of both the empty and full samples, as demonstrated in Table 1.
Table 1. Samples tested with the Vericheck ddPCR Empty-Full Capsid Kit. Source: Bio-Rad Laboratories
| Sample |
Description |
| 1 |
Vendor A full capsids |
| 2 |
50% Vendor A capsids + 50% empty capsids |
| 3 |
Vendor B full capsids |
| 4 |
Vendor C full capsids |
ddPCR assay analysis
All of the samples were examined using the Vericheck ddPCR Empty-Full Capsid Kit, according to the Vericheck ddPCR Empty-Full Capsid Kit User Guide, utilizing a QX200™ Droplet Digital PCR System. For analyses conducted using the CMV promoter and eGFP, the default ITR-2 assay was substituted and tested individually. Every assay and sample combination was tested utilizing triplicates of the antibody binding reaction, with three ddPCR wells for each antibody binding reaction.
ddPCR wells were examined using QX Manager Software, Premium Edition, Version 2.2 (Bio-Rad, #12018108). The kit user guide discusses utilizing the positive control automatic thresholding option to assess the data. Yet, the replaced genome assays might not be present in the positive control supplied in the kit and instead require manual thresholding. The threshold was placed by hand at a quarter of the whole distance between the center of the double-negative cluster and the center of the capsid detection assay cluster and a quarter between the double-negative cluster and the gene of interest assay, as displayed in Figure 1.
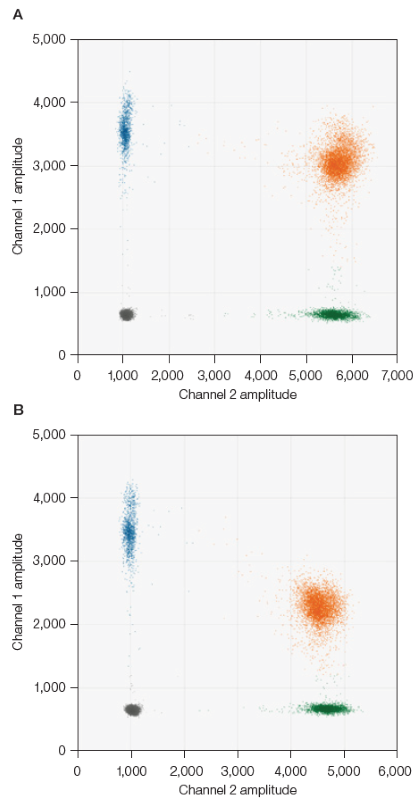
Fig. 1. Example of manual thresholding for genome assays. A, eGFP in HEX. B, CMV promoter in HEX. Capsid detection assay in FAM, channel 1 (●); genome assay in HEX, channel 2 (●); double-positive cluster (●). Image Credit: Bio-Rad Laboratories
Examining the percentage of full capsids
No updates to the analysis discussed in the Vericheck ddPCR Empty-Full Capsid Kit User Guide were required to calculate the percentage total of the genes under investigation. All of the samples were assessed utilizing the Vericheck ddPCR Empty-Full Capsid Analysis Worksheet. The percentage full for each of the genes under investigation was contrasted with the percentage of capsids filled with ITR-2.
Results
Figure 2 displays the capsid titer for each sample utilizing each genome reference. Capsid titer was comparable regardless of which assay was utilized as a genome reference.
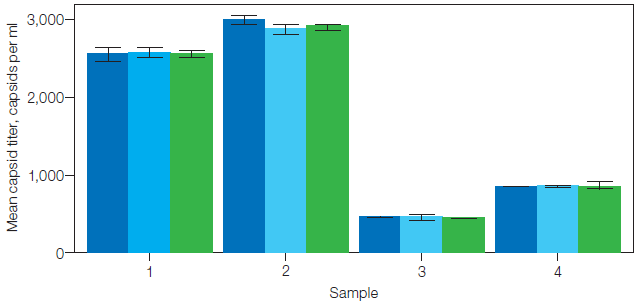
Fig. 2. Capsid titer by sample and genome reference. The concentration of AAV capsids using each genome reference is presented as a bar. Error bars mark one standard deviation. Targets: ITR-2 (■), eGFP (■), and CMV promoter (■). Image Credit: Bio-Rad Laboratories
Figure 3 displays the percentage of filled capsids. Sample 4 resulted in higher variations among targets, suggesting the presence of partial capsids
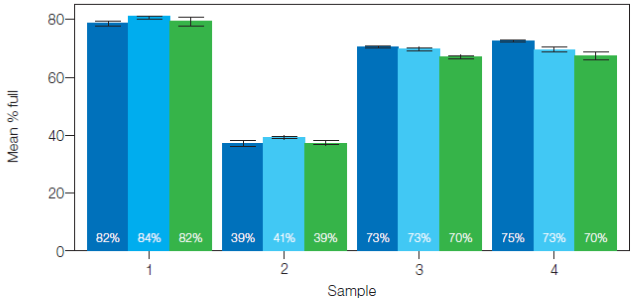
Fig. 3. Percentage of full capsids for all samples. The percentage of capsids filled with each target is presented as a bar. Error bars mark one standard deviation. Targets: ITR-2 (■), eGFP (■), and CMV promoter (■). Image Credit: Bio-Rad Laboratories
Conclusions
When contrasting the genome assays’ findings, all of the assays generated almost identical viral titers for each of the different samples. This outcome suggests that any present AAV genomic target is enough to adjust capsid measurement and that this applies to all fill rates. When assessing samples from various vendors, variations in fill rate by gene were recorded with Vendors B and C, potentially suggesting the presence of partial genomes.
To conclude, this research was successful in expanding the Vericheck ddPCR Empty-Full Capsid Kit to assess the percentage of AAV capsids filled with different genomic targets other than ITR-2. Such an extended capacity can be utilized with different AAV genomes to define partial capsids for a more detailed examination of AAV integrity.
By integrating different genome targets, the kit offers higher degrees of flexibility and awareness in packaging effectiveness, expanding its potential use in gene therapy applications.
About Bio-Rad Laboratories
For over six decades, Bio-Rad has provided the healthcare industry with innovative and useful products that help life science researchers accelerate the discovery process and medical diagnostic labs obtain faster, better results.
Bio-Rad is among the top five life science companies in the world, providing instruments, software, consumables, reagents, and content for the areas of cell biology, gene expression, protein purification, protein quantitation, drug discovery and manufacture, food safety and environmental quality testing, along with science education. Our products and solutions are based on technologies to separate, purify, identify, analyze, and amplify biological materials such as antibodies, proteins, nucleic acids, cells, and bacteria.
As a leading global provider of in-vitro diagnostics supplies, our diagnostic products and systems leverage a broad range of technologies and deliver high-value clinical information in the blood transfusion, diabetes monitoring, autoimmune, and infectious disease testing markets. These products are used to support the diagnosis, monitoring, and treatment of diseases and other medical conditions.
Bio-Rad is the world leader in clinical quality control products, services, and information systems, products that ensure the accuracy and validity of clinical test results.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.