Sino Biological has managed to successfully create a portfolio of GMP-grade cytokines with the help of a GMP-grade quality management system. This system uses excellent quality management and liberating testing criteria.
- Product safety testing system
- Quality assurance management system
- Quality control system
- GMP-grade quality management system
Cytokines - low molecular weight proteins or minor polypeptides - play significant roles in transmitting data between cells and controlling immune responses and effector functions, such as the promotion of cell proliferation and growth and tissue repair.
In basic research in life sciences, they are widely utilized, as well as in cancer immunotherapy, regenerative medicine, stem cell therapy, and chimeric antigen receptor-T cell /natural killer cell preparation, and culture. Thus, stricter standards and regulations have been set to guarantee the safety of cytokines.
GMP-grade cytokines have been manufactured in accordance with GMP standards, which involve a strict quality management system, standardized quality assurance management, and quality control system.
Such systems have been developed to reduce uncertainty and variability in the manufacturing process, thereby ensuring high quality of cytokine products.
Unlike recombinant drugs, GMP-grade cytokines cannot be directly used for disease treatment but can serve as excipients for cell therapy. Although GMP-grade cytokines do not need approval from the United States Food and Drug Administration, during production, strict compliance with GMP is mandatory.
For cell therapy research and clinical applications, Sino Biological is devoted to manufacturing high-quality reagents. By applying a quality management system that sticks to GMP standards, and implementing rigorous quality management and liberate testing criteria, Sino Biological has successfully made a range of high-quality GMP-grade cytokines.
Quality management and quality control system
At Sino Biological, extensive quality control tests are carried out to make sure that all GMP-grade cytokines are in-line with rigorous quality standards.
GMP-grade quality management system
- Complies with ISO 9001:2015 and ISO 13485:2016
- Complies with the Good Manufacturing Practice
- Full regulatory support files
- FDA DMF filed
Quality control system
- Complies with USP Chapter <1043> Ancillary Materials For Cell, Gene, and Tissue-engineered Products
- Complies with the General Chapter of Chinese Pharmacopoeia, 2020 Edition, Volume III
- Complies with USP Chapter <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing
Quality assurance management system
- Quality assurance management system
- Raw material and supplier management system
- Facility and equipment management system
- Document and record management system
- The quality assurance team reviews and approves all documents and records
Product safety testing system
- Mycoplasma testing
- Sterility testing
- Adventitious virus testing
- Abnormal toxicity testing
- Residual host cell protein levels less than 0.5 µg/mg (500 ppm)
- Residual host cell DNA levels less than 100 pg/mg
- Bacterial endotoxin levels less than 5– 10 EU/mg
Quality system certification

Image Credit: Sino Biological US Inc
4,000 m2 of GMP cleanroom facilities
Sino Biological has established over 4,000 m2 of GMP cleanroom facilities and also has eukaryotic and prokaryotic cell facilities that are fitted with advanced culture equipment and purification equipment. This allows the company to have complete control of the full production process.

Bioreactors. Image Credit: Sino Biological US Inc

Purification Equipment. Image Credit: Sino Biological US Inc

Homogenizer. Image Credit: Sino Biological US Inc
Featured GMP-grade cytokines
Source: Sino Biological US Inc
| Cytokine |
Cat# |
Purity |
Expression Host |
Bioactivity (ED50) |
| IL-21 |
GMP-10584-HNAE
DMF Filed
|
≥95% |
E. coli |
Induced Interferon gamma secretion by human natural killer lymphoma NK-92 cells.
The ED50 for this effect is 0.4-2 ng/mL. |
| IL-7 |
GMP-11821-HNAE |
≥95% |
E. coli |
Cell proliferation assay using anti-CD3 antibody activated human peripheral blood mononuclear cell (PBMC). The ED50 for this effect is typically 0.5-8 ng/mL. The specific activity of Recombinant Human IL-7 is >100,000 units/μg, which is calibrated against the human IL-7 reference standard (NIBSC code: 90/530). |
| IL-2 |
GMP-11848-HNAE |
≥95% |
E. coli |
Cell proliferation assay using CTLL2. The ED50 for this effect is typically 1-8 ng/mL. The specific activity of recombinant human IL-2 is approximately 20,000 IU/μg. |
| TNF |
GMP-10602-HNAE |
≥95% |
E. coli |
Cytotoxicity assay using L929 mouse fibrosarcoma cells in the presence of the metabolic inhibitor actinomycin D. The ED50 for this effect is typically 3-30 pg/mL. The specific activity of recombinant human TNF-alpha is >36,000IU/μg, which is calibrated against the human TNF-alpha WHO International Standard (NIBSC code: 12/154). |
| IL-6 |
GMP-10395-HNAE |
≥95% |
E. coli |
Cell proliferation assay using TF‑1 human erythroleukemic cells. The ED50 for this effect is 0.1-0.8 ng/mL. The specific activity of recombinant human IL-6 is >100,000IU/μg, which is calibrated against human IL-6 WHO International Standard (NIBSC code: 89/548). |
| IL-1B |
GMP-10139-HNAE
DMF Filed
|
≥95% |
E. coli |
Induced Interferon gamma secretion by human natural killer lymphoma NK-92 cells. The ED50 for this effect is typically 0.2-2 ng/mL. The specific activity of recombinant human IL-1 beta is >60,000 IU/μg, which is calibrated against the human IL-1 beta WHO International Standard (NIBSC code: 86/680). |
| IL-4 |
GMP-11846-HNAE |
≥95% |
E. coli |
Cell proliferation assay using TF‑1 human erythroleukemic cells. The ED50 for this effect is 0.05-0.25 ng/mL. The specific activity of recombinant human IL-4 is >10,000 IU/μg which is calibrated against the human IL-4 WHO International Standard (NIBSC code: 88/656). |
| IL-15 |
GMP-10360-HNAE |
≥95% |
E. coli |
Measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells. The ED50 for this effect is typically 3-12 ng/mL. |
| IL15 |
GMP-10360-HNCE |
≥ 95 % |
E. coli |
Cell proliferation assay using MO7e human megakaryocytic leukemic cells. The ED50 for this effect is typically 0.5-5 ng/mL. |
| IFN-γ |
GMP-11725-HNAE |
≥95% |
E. coli |
Measured in anti-viral assays using WISH cells infected with vesicular stomatitisvirus (VSV). The ED50 for this effect is 8-80 pg/mL. |
| OSM |
GMP-10452-HNAH |
≥95% |
HEK293 Cells |
Measured in a cell proliferation assay using TF-1 human erythroleukemic cells.The ED50 for this effect is typically 0.3-1.2 ng/mL.The specific activity of recombinant human OSM is >30,000 Units/μg, which is calibrated against the human OSM WHO International Standard (NIBSC code: 93/564). |
| IL12 |
GMP-CT011-H08H |
≥ 95 % |
HEK293 Cells |
Induced Interferon gamma secretion by human natural killer lymphoma NK-92 cells. The ED50 for this effect is typically 0.04-0.4 ng/mL. The specific activity of recombinant human IL-12 is >10,000 Units/μg, , which is calibrated against the human IL-12 WHO International Standard (NIBSC code: 95/544). |
| Activin A |
GMP-10429-HNAH |
≥ 95 % |
HEK293 Cells |
Inhibited proliferation of MPC-11 cells. The ED50 for this effect is 0.5-5ng/mL. The specific activity of recombinant human Activin A is >1,000U/mg, which is calibrated against the human Activin A International Standard (NIBSC code: 91/626). |
| GM-CSF/CSF2 |
GMP-10015-HNAH |
≥ 95 % |
HEK293 Cells |
Cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is typically 0.06-0.3 ng/mL. |
| IL3 |
GMP-11858-HNAE |
≥ 95 % |
E. coli |
Cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is typically 0.15-0.75 ng/mL. |
| FGF10 |
GMP-10573-HNAE |
≥ 95 % |
E. coli |
Cell proliferation assay using BaF3 mouse pro-B cells transfected with human FGFR2b. The ED50 for this effect is typically 3-30 ng/mL. |
| EGF |
GMP-10605-HNAE |
≥ 95 % |
E. coli |
The specific activity of human EGF is >1,000 x IU/μg, which is calibrated against the human EGF WHO International Standard (NIBSC code: 91/530). |
| FGF2 |
GMP-10014-HNAE |
≥ 95 % |
E. coli |
Cell proliferation assay using Balb/c 3T3 mouse embryonic fibroblasts. The ED50 for this effect is typically 0.01-0.1 ng/mL. The specific activity of human FGF basic/FGF2 GMP is >1,000 IU/μg, which is calibrated against the human FGF basic/FGF2 WHO International Standard (NIBSC code: 90/712). |
[Note: Sino Biological, Inc. sells GMP-grade products designed for research, manufacturing use, or ex vivo use and not intended for human in vivo applications.]
GMP grade cytokines: High purity, high bioactivity, high stability, high batch-to-batch consistency
High purity
Human Interleukin-21/IL-21 protein
Cat#: GMP-10584-HNAE
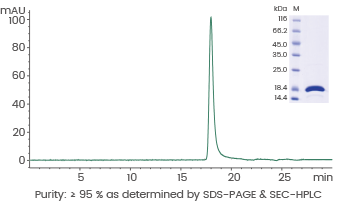
Image Credit: Sino Biological US Inc
Human IL7/Interleukin 7 protein
Cat#: GMP-11821-HNAE
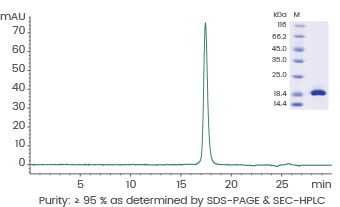
Image Credit: Sino Biological US Inc
Human IL-1 beta/IL1B protein
Cat#: GMP-10139-HNAE
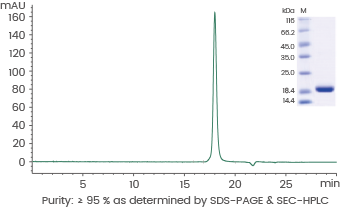
Image Credit: Sino Biological US Inc
High bioactivity
Human interleukin-21/IL-21 protein
Cat#: GMP-10584-HNAE
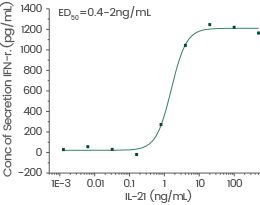
Induced Interferon gamma secretion by human natural killer lymphoma NK-92 cells. Image Credit: Sino Biological US Inc
Human IL7/Interleukin 7 protein
Cat#: GMP-11821-HNAE
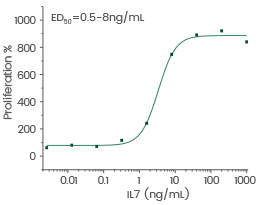
Cell proliferation assay using anti-CD3 antibody activated human peripheral blood mononuclear cell (PBMC). Image Credit: Sino Biological US Inc
Human Interleukin-2/IL-2 protein
Cat#: GMP-11848-HNAE
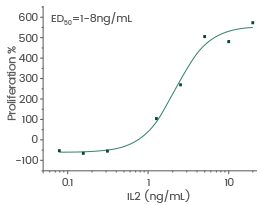
Cell proliferation assay using CTLL2. The specific activity of recombinant human IL-2 is approximately 20,000 IU/μg. Image Credit: Sino Biological US Inc
High stability
Human Interleukin-21/IL-21 protein
Cat#: GMP-10584-HNAE
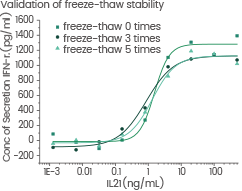
GMP Human IL-21 freeze-thawed 3 times and 5 times without performance reduction. Image Credit: Sino Biological US Inc
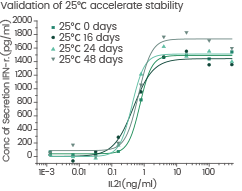
GMP Human IL-21 accelerated at 25 ℃ for 0/16/24/48 day without performance reduction. Image Credit: Sino Biological US Inc
High batch-to-batch consistency
Human Interleukin-21/IL-21 protein
Cat#: GMP-10584-HNAE
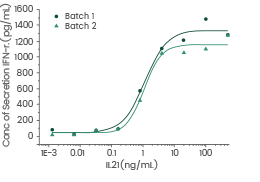
Induced Interferon gamma secretion by human natural killer lymphoma NK-92 cells. Image Credit: Sino Biological US Inc
Human IL7 / Interleukin 7 protein
Cat#: GMP-11821-HNAE
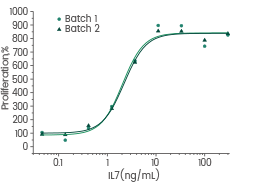
Cell proliferation assay using anti-CD3 antibody activated human peripheral blood mononuclear cell (PBMC). Image Credit: Sino Biological US Inc