Nonalcoholic fatty liver disease (NAFLD) and its more severe form, nonalcoholic steatohepatitis (NASH), are major health concerns. According to the American Liver Foundation, about 25% of adults in the United States have NAFLD, and 5% have advanced to NASH.
As of August 2023, there is no FDA-approved treatment for this condition. Various biomarkers and drug targets, such as Fatty Acid Synthase (FASN), Thyroid Hormone Receptor Beta (THR-β), NR1H4/FXR, Glucagon-Like Peptide-1 Receptor (GLP-1R), Peroxisome Proliferator-Activated Receptor Alpha (PPARA), FGF19, FGF21, and ATP citrate lyase (ACLY), are known to play a role in NASH development due to their dysregulation.
Clinical trials of the THR-β agonist Resmetirom have shown promising results for treating NASH. Ongoing research is also exploring FASN inhibitors to reduce lipids, NR1H4/FXR agonists to address bile acid-related injuries, GLP-1R agonists to improve insulin sensitivity, and FGF19/FGF21 analogs to reduce hepatic steatosis. ACLY inhibitors also show potential in NASH treatment.
Sino Biological provides recombinant NASH-related drug targets to support drug discovery and study the interactions between these targets, offering valuable insights for developing innovative NASH therapies and addressing associated conditions like diabetes.
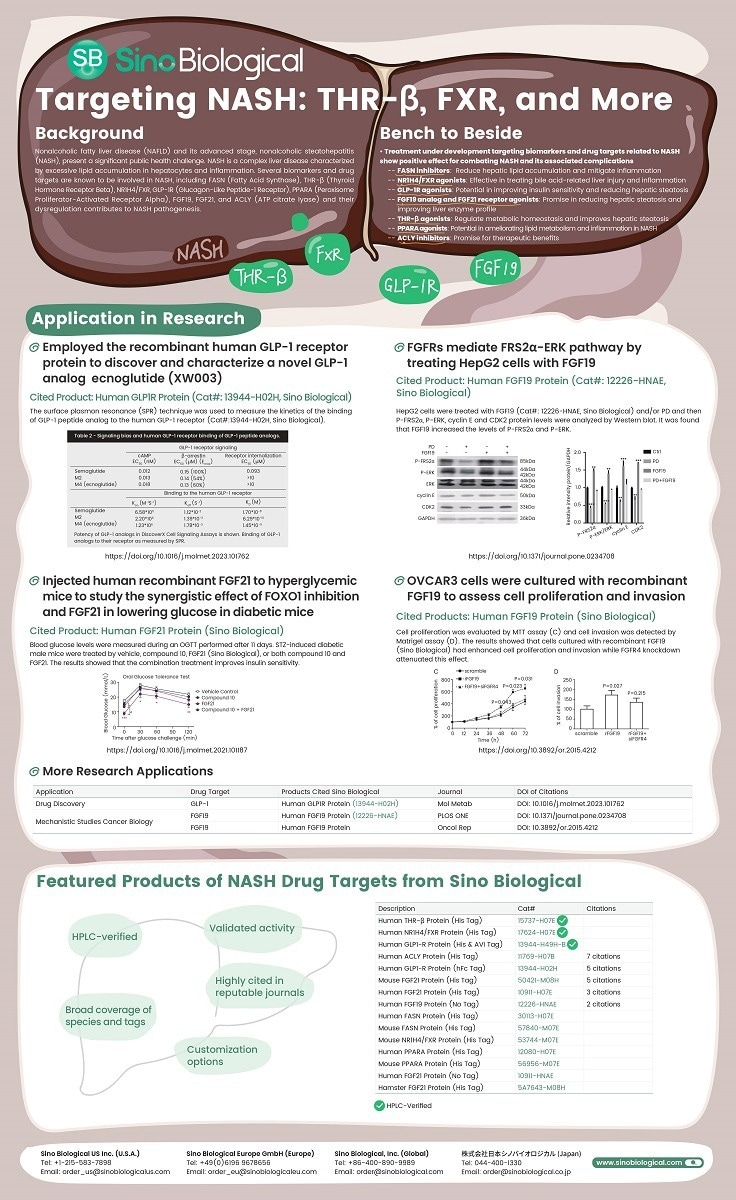
Image Credit: Sino Biological Inc.
Physiological and pathological roles
NASH is a complex liver disease marked by too much fat buildup in liver cells and inflammation. To create precise treatments, it is crucial to fully grasp how NASH-related biomarkers work in both normal and disease states.
For example, when FASN activity is out of balance, it can lead to hepatic steatosis, a key feature of NASH, despite its essential role in liver fat processing. In NASH, disrupted THR-β signaling messes up the metabolic balance, causing more fat buildup and inflammation.
When NR1H4/FXR, a nuclear receptor, does not function appropriately in NASH, it can result in bile acid-related liver damage and inflammation. GLP-1R issues in NASH can lead to insulin resistance and more fat in the liver.
Altered PPARA signaling in NASH contributes to messed-up fat processing and liver inflammation. Importantly, FGF19 and FGF21 are essential targets for drugs that regulate bile acids and energy in the liver during NASH. ACLY might also contribute to excess liver fat by making new fats. Understanding these roles is critical to developing effective ways to treat NASH.
Bench to bedside
Addressing the complex development of NAFLD and NASH is increasingly important due to their growing prevalence. Several biomarkers and drug targets, including FASN, THR-β, GLP-1R, PPARA, FGF19, FGF21, NR1H4/FXR, and ACLY, provide promising avenues for tackling NASH and its related issues.
Inhibiting FASN aims to reduce fat buildup in the liver and reduce inflammation. Preclinical studies show the effectiveness of NR1H4/FXR agonists like Tropifexor and Nidufexor in addressing liver damage and inflammation related to bile acids. GLP-1R agonists like Semaglutide and Liraglutide can potentially improve insulin sensitivity and reduce liver fat.
FGF19 analog (Aldafermin) and FGF21 receptor agonist (Pegbelfermin) also promise to reduce liver fat and improve liver function. In preclinical studies, the THR-β agonist Resmetirom helps regulate metabolic balance and improves liver fat buildup.
PPARA agonists can potentially improve lipid metabolism and reduce inflammation in NASH. Ongoing investigations into ACLY inhibitors like Bempedoic acid hold promise for their therapeutic benefits.
These ongoing clinical trials and research efforts offer hope for innovative and effective therapies to combat NASH and its associated complications, including diabetes.
Targeting Glucose and Lipid Metabolism in NASH: Overview of Drug Actions. Image Credit: Xu, X., Poulsen, K.L., Wu, L. et al.
Applications in research
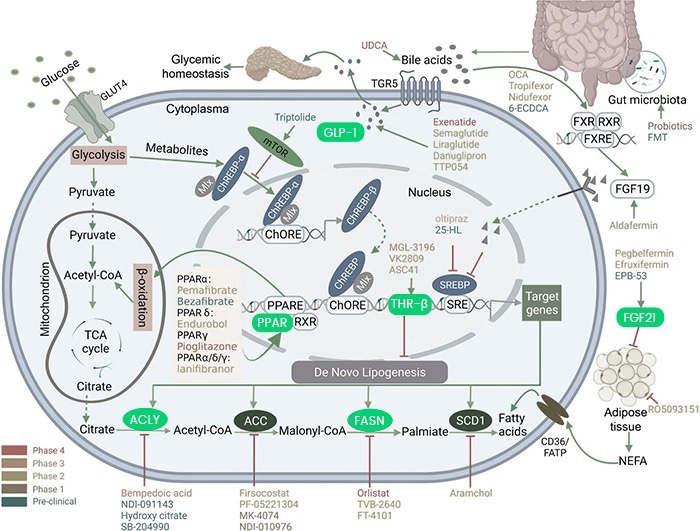
Recombinant drug targets have been crucial in drug development and disease discovery. Guo et al. used the recombinant human GLP-1 receptor protein from Sino Biological (Cat#: 13944-H02H) to discover and characterize ecnoglutide (XW003), a novel GLP-1 analog.
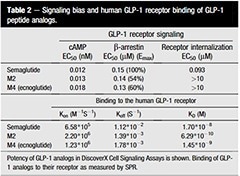
Qiao et al. utilized recombinant FGF19 (Cat#: 12226-HNAE, Sino Biological) to confirm that FGFRs mediate the FRS2α-ERK pathway in HepG2 cells. They found that FGF19 increased the levels of P-FRS2α and P-ERK, shedding light on the signaling mechanisms involved. Hu et al. demonstrated the essential role of FGF19-FGFR4 signaling in ovarian cancer cell proliferation and invasion. They used recombinant FGF19 from Sino Biological to stimulate OVCAR3 cells.
Their results showed that cells cultured with rFGF19 had enhanced cell proliferation and invasion, while FGFR4 knockdown reduced this effect. Lee et al. injected hyperglycemic mice with human recombinant FGF21 from Sino Biological to study the synergistic effect of FOXO1 inhibition and FGF21 in lowering glucose levels in diabetic mice. Their research showed that the combination treatment improved insulin sensitivity.
The surface plasmon resonance (SPR) technique was used to measure the kinetics of the binding of GLP-1 peptide analog to the human GLP-1 receptor (Cat#: 13944-H02H, Sino Biological). Image Credit: Wanjun Guo et al.
HepG2 cells were treated with FGF19 (Cat#: 12226-HNAE, Sino Biological) and/or PD and then P-FRS2α, P-ERK, cyclin E, and CDK2 protein levels were analyzed by Western blot. Image Credit: Chuchu Qiao et al.
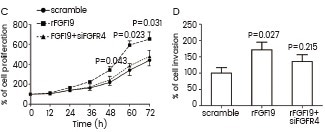
OVCAR3 cells were cultured with recombinant FGF19 (Sino Biological). Cell proliferation was evaluated using the MTT assay (C), and cell invasion was detected using the Matrigel assay (D). Image Credit: Lingling Hu et al.
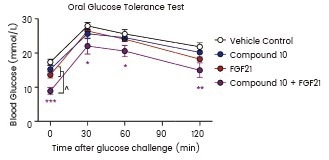
Blood glucose levels were measured during an OGTT performed after 11 days. STZ-induced diabetic male mice were treated by vehicle, compound 10, FGF21 (Sino Biological), or both compound 10 and FGF21. Image Credit: Yun-Kyoung Lee et al.
Featured Sino products
Human THR-β protein (His Tag): (Cat#: 15737-H07E) HPLC verified
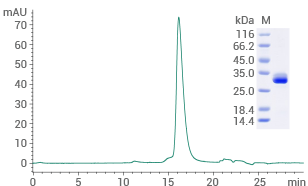
Image Credit: Sino Biological Inc.
Human NR1H4/FXR protein (His Tag): (Cat#: 17624-H07E) HPLC verified
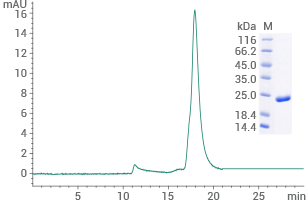
Image Credit: Sino Biological Inc.
Human GLP1-R protein His & AVI Tag (Biotinylated): (Cat#: 13944-H49H-B) HPLC verified
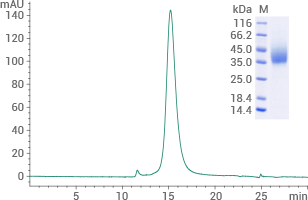
Image Credit: Sino Biological Inc.
Anti-FASN Ab, Rabbit pAb: (Cat#: 100685-T10)
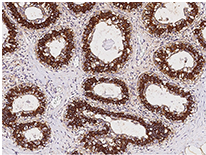
Immunochemical staining of human FASN in the human breast. Image Credit: Sino Biological Inc.
- Applications: IHC-P, ICC/IF
More recombinant NASH drug targets
Source: Sino Biological Inc.
| Product |
Cat# |
Tag |
Citations |
| Human ACLY |
11769-H07B |
N-His |
7 Citations |
| Human GLP1-R |
13944-H02H |
hFc |
5 Citations |
| Mouse FGF21 |
50421-M08H |
His Tag |
5 Citations |
| Human FGF21 |
10911-H07E |
His Tag |
3 Citations |
| Human FGF19 |
12226-HNAE |
No Tag |
2 Citations |
| Human FASN |
30113-H07E |
N-His |
|
| Mouse FASN |
57840-M07E |
N-His |
|
| Mouse NR1H4/FXR |
53744-M07E |
N-His |
|
| Human PPARA |
12080-H07E |
N-His |
|
| Mouse PPARA |
56956-M07E |
N-His |
|
| Human FGF21 |
10911-HNAE |
No Tag |
|
| Hamster FGF21 |
5A7643-M08H |
His Tag |
|