In the past few years, multispecific antibodies, such as trispecific, bispecific, and tetraspecific, have become popular in antibody therapeutics.
Compared to conventional monospecific antibodies, multispecific antibodies have become a primary focus area for biotech and big pharmaceutical companies due to the availability of several benefits, such as improved specificity and targeting ability, as well as improved therapeutic effects.
So far, the FDA has approved several bispecific antibody drugs worldwide, and over 100 multispecific antibodies are being examined in clinical trials.
Sino Biological provides an extensive range of products and services to offer customers with entire multispecific antibody development solutions covering several phases of the development process. This includes druggability assessment, animal model evaluation, antibody development and optimization, and process development.
Sino Biological enables customers to expedite their multispecific antibody development and clinical research using such solutions.
Mechanism of action of multispecific antibody drugs
As per the “Bispecific Antibody Development Programs Guidance for Industry” issued by the FDA in May 2021, the mechanism of action of bispecific or multispecific antibodies could be primarily divided into the three categories listed below:
- Bridging cells: The antibody bridges immune cells with tumor cells, thereby recruiting and triggering immune cells to kill tumor cells and obtain the redirection of cytotoxic effector cells.
- Bridging receptors: Multiple signaling pathways are involved in the development of tumors; thus, blocking a single signaling pathway cannot entirely curb disease progression. Bispecific antibodies have the potential to trigger or inhibit multiple signaling pathways concurrently, thereby maintaining coordinated effects.
- Bridging factors: The availability of the bivalent structures of bispecific antibodies mediates the development of protein complexes and membrane receptor protein complexes, thereby increasing the activity of antibody–drug conjugates or agonistic antibodies and yielding equivalent biological effects.
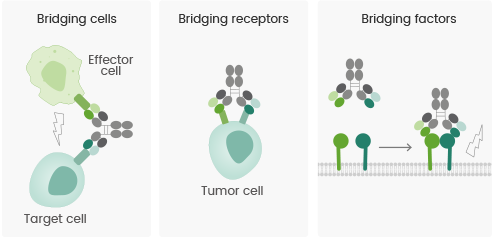
MOA of Multispecific Antibodies. (Reference: doi: 10.1038/s41573-019-0028-1). Image Credit: Sino Biological US Inc
Our solutions for multispecific antibody development
Antibody development
Antibody discovery is considered the starting point for developing multispecific antibody drugs, and its outcomes are of utmost significance. Early drug candidates get to experience the complete process of multispecific antibody drug development and directly identify the attributes of the drug molecules.
Sino Biological provides four major antibody development platforms and four major protein expression platforms, which enable engagement in early development projects of multispecific antibody drugs and fulfill the various development needs of industrial and scientific customers.
Four antibody development platforms
The structure of multispecific antibodies causes an impact on their biological activity, thereby making it a vital aspect of their development. Sino Biological provides four significant antibody development platforms: a FACS single B-cell sorting platform, a phage-display platform, the Beacon® single B-cell platform, and a hybridoma development platform.
Such platforms cover all processes, from antigen design and preparation to animal immunization, via antibody production, enabling Sino Biological to screen and determine antibody molecules that fulfill customers' expectations.
Phage display platform
Sino Biological offers phage display screening services and antibody library construction for several species, such as rabbits, mice, and chickens.
- Experience in producing over 5,000 rabbit mAbs
- Successful screening rate of 90%
FACS single B cell sorting platform
The FACS B cell sorting platform offers high-throughput, specific, efficient, and quick production of naïve paired light and heavy chain variable regions.
- All B cells could be sorted within one day
- B-cell culture for 10 to 12 days
Beacon® single B cell screening platform
The Beacon® platform automatically screens around 10,000+ plasma cells, briefing the screening process from 1 to 2 months to a single day.
- As rapid as 35 days to achieve positive clones
- Screening over 10,000 B cells at a time
Hybridoma development platform
Sino Biological comes up with customized animal immunization, antigen design, clone screening, and antibody identification solutions for customers.
- Project success rate is more than 90%
- Mouse mAb affinity goes up to the pM level.
Protein expression service
The design of differential antigens raises the probability and success of producing differential antibodies via screening. The choice of antigens and their quality are directly linked to the successful production of antibodies with utility and specificity.
Drawing from 15 years of experience in protein research and development, Sino Biological offers four major expression systems for mammalian cells, insect cells, E. coli cells, and HEK293/CHO stable cell lines. This enables the development of different antigens types and provides customers with high-quality proteins.
Mammalian transient platform
Using CHO and HEK293 cells as hosts, Sino Biological allows high-yield and high-throughput expression of recombinant proteins and antibodies.
- 10,000+ proteins and antibodies expressed and purified successfully
- Adaptable culture volumes: 3 mL to 1,500 L
Baculovirus-insect platform
Having improved expression vectors and virus packaging technology, Sino Biological offers high-quality recombinant expression and purification services.
- Recombinant protein purity >95%;
- 1,000+ proteins successfully expressed and purified
Bacterial protein expression platform
Sino Biological provides a one-stop solution for services varying from codon optimization to recombinant protein expression and purification.
- Quick protein delivery within three weeks
- 1,000+ proteins expressed and purified successfully
Stable cell line development
Sino Biological has ample experience connected stable cell line development, such as raising cell productivity and enhancing cell status.
- Stable cell lines with antibody yields of up to 2 to 4 g/L
- Provided 15+ years of experience
Antibody optimization
As soon as candidate antibodies are obtained from screening, they need optimization and modification to guarantee the quality of the multispecific antibody drugs. Common antibody optimization strategies include affinity maturation, antibody humanization, and stability enhancement.
In protein expression and antibody development and production, based on years of experience, Sino Biological offers one-stop services for antibody humanization, multispecific antibody preparation, AI-Powered affinity maturation, and thus providing customers with a range of improved modifications and providing high-quality antibodies to assist their research and development measures.
Antibody humanization service
Antibodies gathered from hybridomas or murine ImmunoBanks are not known to be ideal for direct clinical use, as they could evoke immunogenic and toxic side effects in humans. For this problem to be overcome, humanization modifications are needed.
With the help of complementarity-determining region grafting technology and computer-aided molecular modeling, Sino Biological offers highly efficient and trustworthy antibody humanization services that have the potential to obtain a high degree of successful humanization (>95%).
- Success rate is 100%
- Affinity validated by ELISA/SPR or BLI
- As quick as 3 to 4 weeks to provide preferred humanized antibodies
AI-Powered affinity maturation service
The antibody drugs’ potency and efficacy rely heavily on their affinity. Normally, the affinity of humanized or murine antibodies does not fulfill the standards required for therapeutic use; hence, affinity maturation is warranted.
Sino Biological applies an AI-assisted platform for high-throughput affinity maturation and HTP antibody expression, enabling quick and efficient screening. The platform has been validated to obtain a 103-fold increase in antibody affinity.
- As quick as 4-6 weeks to delivery
- It is possible to increase affinity by 103 times
- Up to 1010 sequence space can be screened
Multispecific antibody production service
Based on the mammalian cell expression platform, Sino Biological offers antibody expression services in the microgram-to-gram range. The company uses efficient expression vectors, transfection reagents, an improved proprietary medium, and high-density cell suspension culture technology to fulfill customers' various application needs.
- Multiple purification methods: Protein A/G/L, SEC IEC,
- High quality delivery: SEC-HPLC > 95%, endotoxin < 1 EU/mg
- Around 14 types of multi-specific antibody expression experience
Comprehensive multispecific antibody evaluation service
In vitro efficacy evaluation could offer data for the early screening of multispecific antibodies. Sino Biological has fixed an in vitro activity assay platform for antibody drugs. The platform has been developed to assist customers' multispecific antibody drug development projects and allows the effective completion of in vitro efficacy assays.
- ISO 9001 & CNAS certified, robust quality management system
- With extensive experience, can provide an SPR/BLI affinity assay and an antibody-binding activity assay
- Comprehensive services and reagents, multi-dimensional efficacy evaluation system
Druggability assessment
During the early stage of molecular screening, druggability is a main property of the molecule and is primarily determined. It is vital to consider druggability as it could affect the cost of the following development process and the risks involved at the time of the clinical stage.
Hence, druggability assessment significantly links drug discovery and drug development. To assist customers in choosing the best candidates, Sino Biological has fixed an in vitro efficacy evaluation platform.
Furthermore, the company offers high-quality cytokines and protein expression services for cell proliferation inhibition assays and the mixed-lymphocyte reaction (MLR) assay service, thus assisting the druggability evaluation of multispecific antibody drugs.
Special protein expression service
For the druggability of antibodies to be evaluated, it is essential to guarantee their particular affinity to their antigens. This needs the choosing of the correct antigen for the antibody development project.
A range of recombinant protein expression systems has been offered by Sino Biological, which has been customized to fulfill varied research requirements, thereby increasing the probability of obtaining successful outcomes in research projects.
- Four expression systems: E. coli, insect, mammalian, and CHO/HEK293 stable cell line development
- HTP expression ability, assisting the quick breakthrough of multispecific antibody drugs
- Comprehensive QC testing: SDS-PAGE, FACS, SEC-HPLC, ELISA, cell assays, BLI/SPR, etc
Comprehensive multispecific antibody evaluation service
Guaranteeing the efficacy and safety of drugs is vital for the successful development of new pharmaceuticals. Thus, functional tests are a vital idea of druggability evaluation. Sino Biological has fixed an in vitro activity assay platform for antibody drugs, providing antibody-binding activity assay and SPR/BLI affinity assay services. These evaluations help identify antibodies' binding affinity and specificity to their target proteins.
- Wide experience, strong, and reliable methodology
- ISO 9001 and CNAS certified, strong quality management system
- Extensive services and reagents, multi-dimensional efficacy evaluation system available
High-quality cytokines and ELISA pair sets
Cytokines are extensively utilized in MLR assays and cell proliferation inhibition assays. Cell proliferation inhibition assays are vital for identifying the biological activities of antibody drugs.
In return, MLR assays are especially beneficial for assessing the effect of test drugs on human primary T-cell functions and for examining the antigen-presentation effect of test drugs in T-cell activation mediated by primary APCs.
Sino Biological provides a range of cytokines with high purity, activity, and inter-batch consistency, like VEGF165, M-CSF, IL-3, and IL-4, extensively utilized in MLR assays and cell proliferation inhibition assays.
Besides, Sino Biological has developed a series of high-quality ELISA Pair Sets for IL-4, IL-6, IL-8, and IFN-gamma, which could be utilized to quantify the side effects of antibody drugs. For instance, binding antibody drugs to lymphocyte Fc receptors triggers the release of cytokines and ADCC.
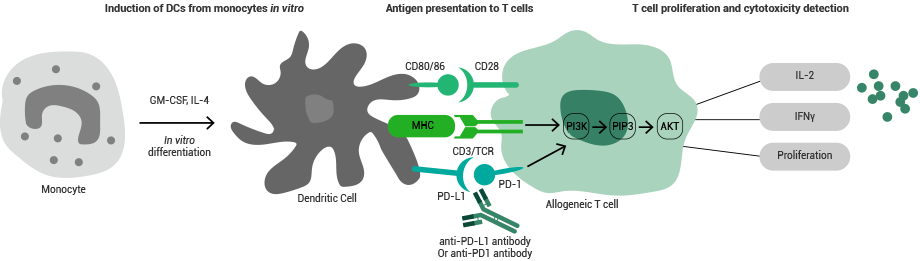
Principle of the MLR assay. Image Credit: Sino Biological US Inc
More highly active cytokines
Source: Sino Biological US Inc
| Molecule |
Cat# |
Species |
Purity |
| M-CSF/CSF1 |
11792-HNAH |
Human |
>85% |
| VEGF165 |
11066-HNAH |
Human, Cynomolgus |
>95% |
| CSF1R |
10161-H02H |
Human |
>95% |
| IGF1 |
10598-HNAE |
Human |
>95% |
| IL-3 |
11858-HNAE |
Human |
≥95% |
| IL-4 |
11846-HNAE |
Human |
≥95% |
| GM-CSF/CSF2 |
10015-HNAH |
Human |
≥95% |
More ELISA pair sets
Source: Sino Biological US Inc
| Target |
Cat# |
Species |
Linear Range (pg/mL) |
| IL-4 |
SEKA11846 |
Human |
10.94-700 pg/mL |
| IL-6 |
SEKB10395 |
Human |
3.13-200 pg/mL |
| IL-8 |
SEK10098 |
Human |
3.75-240 pg/mL |
| IL-10 |
SEKA10947 |
Human |
14.06-900 pg/mL |
| IL-12 |
SEKCT011 |
Human |
31.25-2000 pg/mL |
| IFN-gamma |
SEKA11725 |
Human |
21.88-1400 pg/mL |
| TNF-alpha |
SEKA10602 |
Human |
23.44-1500 pg/mL |
Animal model evaluation
To guarantee the successful preclinical development of multispecific antibodies, fixing animal models to assess their efficacy, safety, and pharmacokinetics is vital. But the performance of animal experiments and functional validation needs the production of various candidate antibody drug molecules.
Based on years and expertise in custom antibody production, Sino Biological offers large-scale multispecific antibody production services for preclinical antibody drug production.
Besides, the company offers PK or anti-drug antibodies (ADA) antibody preparation services, immunogenicity assays for multispecific antibodies, and serum concentration analysis, thus assisting pharmacodynamic experimental design and candidate molecule evaluation.
Large-scale multispecific antibody production service
Sino Biological has fixed a large-scale recombinant antibody expression platform that accommodates the requirement for high-throughput and large-scale production of antibody drugs.
The platform provides recombinant antibody production services in the milligram to kilogram range. The ISO-certified manufacturing facility guarantees that all antibody production happens in a certified environment, thus guaranteeing high-quality products for customers.
- Scalable production capabilities: culture volumes alter from shake flasks to 1500 L bioreactors.
- High quality delivery: SEC-HPLC > 95%, endotoxin < 1 EU/mg
- Around 80+ bioreactors: concurrently assisting dozens of antibody projects.
PK/ADA antibody preparation service
Regarding the development of multispecific antibody drugs, anti-idiotype antibodies play a vital role in detecting and quantifying antibody-drug levels in human or animal serum.
This offers them the necessary detection reagents for PK studies. Besides, they act as a significant reference standard available for immunogenicity analysis.
For drug development to be expedited, Sino Biological offers an extensive range of services, such as anti-idiotype antibody development, antigen preparation, detection method establishment, and kit development.
- Five Antibody Development Platforms: hybridoma, FACS B cell, Beacon®, phage display, and pAb technologies
- High Quality: high titer, low cross-reactivity, high specificity, and with human IgG
- Multiple Purification Methods: Protein A, total human IgG, antigen affinity, Isotype IgG
- Rich Experience: serving clients across the globe with success rate >95%
Process development clinical studies
To assist the clinical research and process development of multispecific antibodies, Sino Biological offers an extensive range of products and services, such as ADA antibody development service, PK antibody development service, cell line development service, and CHO cell culture media.
Cell line development service
Multispecific antibody CMC development starts with the generation of effective and stable cell lines, and it directly identifies the process complexity, production cost, and entire product quality.
Beginning from the antibody sequence or customer’s cDNA, Sino Biological offers cell line development services varying from expression vector construction to screening for stable cell lines and high-yield.
- Officially licensed CHO-K1 cell lines to assist NDA, IND, and BLA applications.
- Comes with a range of service packages
- High yield, with an expression level of no below 2 to 4 g/L
- Quick delivery: as rapid as 8 weeks from gene to purified product
PK antibody development service
During drug development, PK studies are vital for assessing the safety and efficacy of multispecific drugs. Sino Biological provides a range of anti-idiotype antibody generation service packages for quantitatively testing drug levels in animal or human serum. This eases clinical PK and preclinical studies of multispecific drugs.
- Four antibody development platforms: phage-display, hybridoma, Beacon® single B-cell, and FACS single B-cell platforms.
- One-stop service available from antibody development, antigen preparation, and ELISA kit generation via to analytical method development.
- Success rate of more than 95%, serving customers across the globe
- High quality: high sensitivity and specificity
ADA antibody development service
Anti-drug antibodies (ADAs) produced in the body might have an effect on the pharmacodynamic and pharmacokinetic properties of multispecific drugs. Sino Biological has developed high-specificity, high-affinity, and high-sensitivity ADA antibodies for customers, thus assisting the immunogenicity evaluation of multispecific drugs in preclinical and clinical studies.
- Well-established rabbit pAb technology, provides quick and standard rabbit pAb production packages
- Several purification methods: Protein A, total human IgG, antigen affinity, Isotype IgG
- Rich experience: serving clients across the globe with success rate >95%
- High quality: high specificity, high titer, and low cross-reactivity with human IgG
CHO cell culture media
The SMM CHO-GSI medium and SMM CHOGS-C3 medium developed by Sino Biological are ideal for large-scale suspension culture, clone screening of GS-deficient CHO-K1 cells, and stable expression of recombinant antibodies and proteins.
Such media formulations are perfect for assisting the development and production of multispecific drugs, thereby offering trustworthy and effective solutions for biopharmaceutical companies.
- Animal-component-free, serum-free, and antibiotic-free media available
- Suggested for clone screening and suspension culture of GS-deficient CHO cells.
- Strict quality control and stable product batches offered
- Assisting high-cell-density growth and high expression of recombinant proteins.
Additional products and services
- Various species: human, cynomolgus, rhesus macaque, mouse, etc
- High quality: high activity, purity, and batch-to-batch consistency
- Various tags: Avi and His, Fc-Flag and mFc, Fc and Avi, His and Flag, hFc, etc
- Activity validated by ELISA and cell-based assays
- HTP and large-scale production abilities, with the quantity delivered ranging from μg to g
- High-quality delivery: low endotoxin (<1 EU/mg), high purity (SDS-PAGE and SEC-HPLC >95%)
- High bioactivity has been validated by ELISA/SPR/BLI
- High purity: SDS-PAGE ≥ 95% and SEC-HPLC ≥ 90%
- Offering site-specific biotinylated proteins (Avi-tag)
- Expressed in HEK293/CHO cells, akin to the native structure
M13 Mouse mAbs
- High sensitivity and specificity
- HRP, PE, FITC, Biotin, and other labels
- ELISA, WB, FACS, and other applications
Multispecific antibody webinar
Development of multi-specific therapeutic antibodies via computer aided design
Video Credit: Sino Biological