The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused well over 109 million infections and more than 2.4 million deaths. However, there are no approved pharmaceuticals that have shown potent specific activity against the virus, hindering control of the outbreak.
A new study explores the role of common probiotics in managing the disease, which could prove to be of great value, given the emergence of new variants and the broad spectrum of clinical disease in COVID-19.
Probiotics in viral infection
Probiotics are “nonpathogenic living microorganisms providing various health benefits to the human host.” These include Lactobacillus and Bifidobacterium genera of bacteria, typically present in fermented food. Traditionally prepared cuisine often includes such fermented foods, since these bacteria restore the balance of microbes in the gut.
Over the last two decades, many studies and clinical trials have suggested that probiotics may help modulate the immune response and treat various diseases, especially viral infections. Many findings indicate that such probiotics maintain a healthy host immune system that helps the body rebound after a respiratory viral infection in animal models. Not only did these interventions enhance the health of the animals, but lowered the viral load in their lungs and boosted survival rates.
The current paper, which appeared online in the Nutrition Research journal, reviewed the evidence for use of probiotics in preventing viral infections. They arrived at a rough list of probiotic strains that may help prevent infection, and enhance immune function to reduce the impact of viral infections, especially COVID-19.
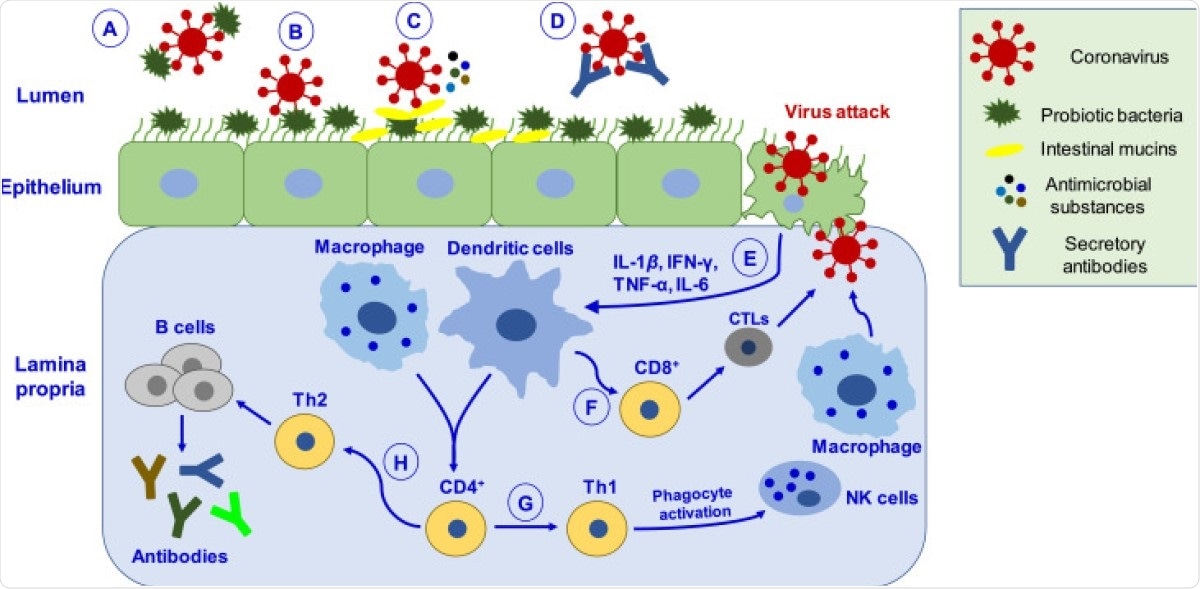
Schematic depiction of putative mechanisms by which probiotics may help manage coronavirus infection. ( A ) Probiotic bacteria can hinder the adsorption process via directly binding to the virus and inhibiting entry into epithelial cells. ( B ) Binding of probiotic bacteria to the epithelial surface can cause steric hindrance and block the virus’s attachment to the host cell receptor. ( C ) Probiotic bacteria releases antimicrobial substances (such as bacteriocins, biosurfactants, lactic acid, hydrogen peroxide, nitric oxide, organic acids) and intestinal mucins from mucosal cells, which can effectively inhibit virus proliferation. ( D ) Virus neutralized by secretory antibodies like IgA. ( E ) Upon virus attack in epithelial cells, probiotics mediate their antiviral effects by eliciting immune responses by activating macrophages and dendritic cells. ( F ) Activation of immune response leads to differentiating CD8 + T lymphocytes into CTLs, capable of destroying virus-infected cells. ( G ) CD4 + T lymphocytes cells differentiate into Th1, which activates phagocytosis through NK cells and macrophages, promoting pathogen killing. ( H ) CD4 + cells differentiate into Th2 cells, which induce B-cells’ proliferation that produces antibodies like IgA, IgG, and IgM. CTLs, cytotoxic T-lymphocytes; Th1, T-helper cells type 1.
Effects of probiotics on host and viral biology
The SARS-CoV-2 virus may be transmitted even in asymptomatic individuals, or during the presymptomatic phases of infection. The latter may last for up to two weeks, exposing others to heavy viral loads and a high risk of infection.
Probiotics may trap the virus in respiratory infection, as well as inhibit binding of the virus to the host cell receptor.
Viral clearance and increased survival
For instance, one mouse model showed that probiotics promoted influenza virus clearance and neutralizing antibody production, via T-helper cells type 1 (Th1). As a result, the virus was cleared from the lungs and other sites of infection.
Another study showed that influenza fatality rates dropped from 100% to 60% when dead and live probiotics, respectively, were administered, and from 60% to 30% when the intranasal route was used. This study showed increased secretory IgA production, and lower proinflammatory cytokine production following infection.
The same researchers also demonstrated the potential efficacy of Lactobacillus species to protect against viral infection. This protection was attributed to their immunomodulatory activity. The same trend was observed in other studies employing Lactobacillus as probiotics in mice infected with influenza viruses.
Similar benefits were found with the Bacillus genus, with inhibition of viral replication and a higher survival rate. The antiviral peptide P18 was found to protect mice in 80% of infections, and reduced the lung viral loads, indicating its potential for further development.
Protective heat-killed probiotics
An interesting study showed that the intranasal use of heat-killed probiotic strain L. casei DK128 helped protect against the influenza virus strain H3N2. All the infected mice survived, while pro-inflammatory cytokine levels dropped, and the viral loads of infected mouse lungs were reduced. This represents another promising avenue of development.
Immunomodulatory effects
Immunomodulatory molecules are active during early SARS-CoV-2 infection, including pro- and anti-inflammatory cytokines. Other players include natural killer (NK) cells and cytotoxic T cells, as well as humoral immune responses.
A combination of three Lactobacillus strains induced an antiviral response, increasing the production of inflammatory cytokines and upregulating interferon regulatory factor-7 and other immunomodulatory genes.
Another team showed that the antiviral effects of probiotic administration could be due to the suppression of a specific protein that mediates virus replication, namely, the SWI2/SNF2-related CREB-binding protein activator protein (SRCAP).
Yet another study demonstrated that probiotics could activate balanced Th1/Th2 responses. These agents were found to prevent infections with other viruses, like Hepatitis C virus, Herpes Simplex Virus type 1, Human immunodeficiency virus.
Protective effects in human viral infections
In human studies, probiotics were found to protect against common cold and flu by over 50%, as well as increasing interferon-gamma (IFN-γ) levels in serum and secretory IgA in the gut. This suggests that probiotics are safe and effective against respiratory infections.
In infants, too, the daily administration of probiotics from birth to one year was associated with a 28% lower risk of recurrent respiratory infections. Similar results were reported for acute infectious disease in infants put on probiotics.
Probiotic consumption seems to improve gut epithelial barrier integrity and regulates inflammatory responses via diverse signaling pathways.
Mechanisms of benefit
The beneficial effects of probiotics are mediated by multiple mechanisms, including the inhibition of bacterial adhesion, better functioning of the mucosal barrier, and modulation of the immune response.
SARS-CoV-2 transmission occurs via respiratory droplets, with the viral spike protein mediating binding to the host cell receptor, the angiotensin-converting enzyme 2 (ACE2). This facilitates viral entry, with local inflammation, followed by systemic inflammatory phenomena and damage to multiple organs.
This damage is often associated with oxidative stress with free radicals causing damage to the cell membranes. COVID-19 patients show high levels of inflammatory cytokines in the blood, often called a cytokine storm. This is thought to be the underlying reason for the severe multi-organ damage occurring in severe COVID-19.
COVID-19 is also linked to dysbiosis of the gut, with a resulting increase in pathogenic bacteria in the gut. This, together with the excessive viral load in the mucosa, disrupts the barrier function of the gut epithelium, with chronic illness. Probiotics may correct the dysbiosis while reducing the viral load and inflammation.
What are the implications?
Strengthening host immunity is among the most effective ways to reduce the severity of COVID-19. Probiotics appear to offer a useful and plausible way to accomplish this.
The investigators conclude that the most useful probiotic strains – including L. gasseri SBT2055, L. casei DK128, B. subtilis 3, L. rhamnosus CRL1505, and B. bifidum strains – showed high potential for further development as anti-COVID-19 therapies.
These were associated with 50% to 80% enhanced survival, and a powerful anti-inflammatory response. Strains like L. rhamnosus GG, L. casei, L. plantarum, L. casei strain Shirota, B. lactis Bb-12, and B. longum brought down both upper respiratory infections, flu-like symptoms and antibiotic-associated diarrhea by 40% to 70%.
Still others, such as L. reuteri ATCC 55730, L. paracasei, L. casei 431, L. fermentum PCC, and B. infantis 35624, were found to have a key immunomodulatory role in various infections.
The utility of such probiotics in COVID-19 may lie in their ability to reduce the risk of infection of the respiratory tract by binding to the virus, or to the epithelial surface itself. This prevents the attachment of the virus to the epithelial cell receptors, often by steric hindrance.
Secondly, they improve the function of the gut epithelial barrier and correct dysbiosis, along with the release of multiple peptides and other molecules that may suppress viral replication. Secretory IgAs may also neutralize the antibodies.
Thirdly, they can modulate the cytokine storm associated with severe and critical disease, and balance the Th1/Th2 responses. Along with their other effects on the immune responses of the host, this can lead to the activation of cellular immunity and specific antibody responses against the virus.
Finally, their powerful antioxidant activity leads to the neutralization of free radicals, which mitigates organ damage. Further studies should identify the optimum combination of such strains, with the effect of each on SARS-CoV-2 biology, refined by other host factors such as age, lifestyle and dietary habits.
Once these findings are validated, probiotics can be introduced into clinical use in the management of COVID-19.