The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a primarily respiratory virus. It is the causative agent of the ongoing coronavirus disease 2019 (COVID-19) pandemic. In severe or critical cases, however, this virus is known to trigger rapid and severe deterioration of multiple organs, including the central nervous system.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Neurodegeneration and COVID-19
Anosmia, or the loss of smell, is a relatively common symptom in COVID-19, but also in Parkinson's disease before motor symptoms set in. This finding, in common with the recently reported occurrence of Parkinson's disease (PD) at a younger age than usual, following SARS-CoV-2 infection, has raised suspicions of a link between these two conditions.
Earlier too, the Spanish flu pandemic of 1918 was followed by an outbreak of a neurological condition called encephalitis lethargica, and subsequently by many cases of post-encephalitic Parkinsonism. Many researchers have turned up links between viral illnesses and PD.
Neurodegenerative illnesses are often characterized by protein aggregates, such as tau and beta-amyloid (Aβ) peptides in Alzheimer's disease (AD) and α-synuclein (αS) in PD, which form amyloid deposits. The aggregation process begins to spread from one cell to another, and brain function is affected.
The link between neurodegeneration and viral infection is unclear. Direct causation has been suggested by the occurrence of Aβ peptide aggregates in cell lines and in animals infected with herpes simplex and respiratory syncytial virus.
Study aims
The current study explores the effect of some proteins of SARS-CoV-2 on αS aggregation and its eventual outcome as amyloid fibril formation.
The spike and nucleocapsid proteins (also known as the S and N proteins) are the most abundant structural viral proteins found in this infection, with the latter being found to have ~1,000 copy numbers per virus particle. The N protein has a net positive charge which allows the viral genome, with its negative charge, to be packed into higher-order structures.
Though the spike protein mediates infection by engaging the host cell receptor, the N protein is also a vaccine target because of its highly conserved nature and greater stability.
αS aggregation with N protein
The researchers found that in vitro, the aggregation of αS showed no change in the presence of spike protein over more than 240 hours of observation. However, in the presence of the N protein, aggregation was of rapid onset, beginning within 24 hours. On further observation, the rate of aggregation changed to reach a second plateau.
With higher N protein concentrations, the lag time to αS aggregation decreases still further, confirming that the N protein interacts with αS leading to its aggregation.
These proteins bear a positive and negative charge, indicating that electrostatic interactions likely occur between them. With a higher salt concentration, these interactions are masked, and this prolongs the onset of aggregation. Even so, its lag time remains much shorter than in the absence of the N protein and remains concentration-dependent.
With the maximum salt concentration, the lag time was still less than that for αS alone, indicating that other interactions are operating, and not just electrostatic forces.
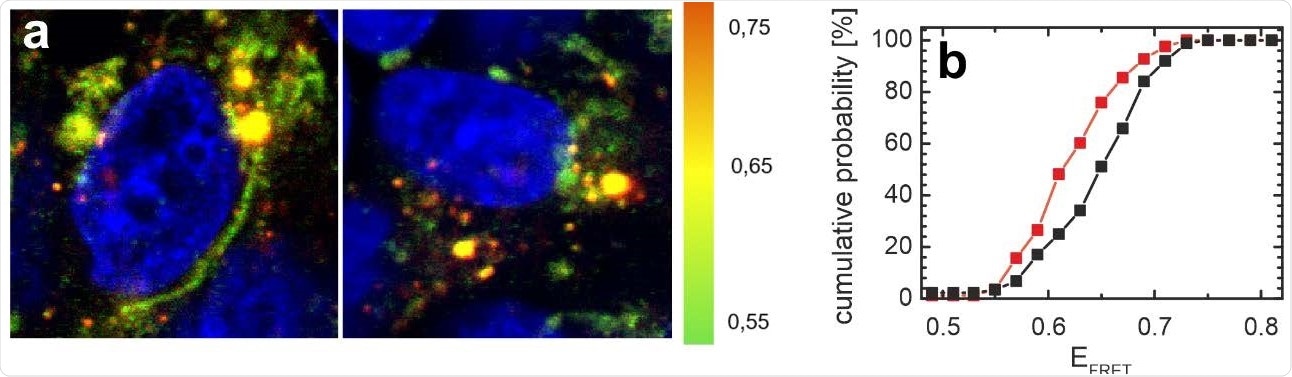
Distribution of αS in SH-SY5Y cells is affected by N-protein. a) FRET images of cells microinjected with the αS FRET probe. The color coding represents EFRET (green: low EFRET; yellow: mid EFRET; orange: high EFRET). The cell nucleus is counterstained with DAPI and visible in blue. A representative image of cells co-injected with N-protein and the αS FRET probe is shown on the left, the control cells on the right were only injected with the αS FRET-probe. b) Distribution of average FRET efficiencies of αS per image for all cells injected with N-protein (red) and control cells (black). The cumulative histograms contain data from at least 80 images for both the control and the N-protein injected samples. The average FRET efficiency of αS in cells injected with N-protein is shifted to lower EFRET values.
Two-stage αS aggregation
The researchers used a technique called microscale thermophoresis assay, which detects changes in the diffusion coefficient of particles on binding to another. This showed an abrupt change from free to bound αS, indicating cooperative binding was at work.
They also used fluorescence correlation spectroscopy (FCS) experiments to explore the number of αS within the αS/N complexes. This showed that at higher N concentrations, slow diffusion occurred because of complex formation. With αS alone, only one molecule is shown to diffuse.
As the N protein concentration rises, a second diffusing molecule appears, which has a much slower diffusion coefficient. The average brightness indicates that the αS in the complexes is almost twice as bright as that of the free αS.
They concluded that 3-4 αS is present per complex, even at N protein excess. This indicates that when αS is in excess, even higher numbers may accumulate on the N protein.
Amyloid fibril-like morphologies
Two plateaus in fluorescence intensity are seen with longer observation periods, with the plateau being higher than the first plateau. Exploring this with atomic force microscopy (AFM), the researchers found amyloid fibrils in a helical form, but with different shapes in the two plateaus.
The first showed two types of fibrils where the helix turns are of different lengths. The second plateau shows homogeneous fibrils of 130 nm, like one of the fibril morphologies in the first plateau. This is concordant with expected αS amyloid fibrils formed without N protein.
In other words, the N protein accelerates αS amyloid fibril formation but preserves their morphology intact.
The αS protein is one with an intrinsic disorder found on membrane vesicles, and its primary function is probably membrane remodeling, related to memory tasks. In its membranous location, it has an α-helical structure.
Altered proteostasis
Using a method called Förster resonance energy transfer (FRET), the investigators looked at how αS is redistributed within a neuronal cell line. This showed that within a similar period, cells which had received a microinjection of N were twice as likely to be dead than the controls, which had 10% dead cells.
In controls, the αS is distributed between the vesicles and the cytosol, with the former having a high FRET value. After microinjection of N protein, an apparently similar distribution is observed, but with less high-FRET signal, indicating a perturbation of αS proteostasis, and therefore a decline in vesicle-bound αS.
Thus, the N microinjection leads to αS redistribution between various conformational states as they lose their function. However, the N protein does not interact with αS in the N-terminal domain, or else its binding here fails to elicit any conformational change in αS. The disturbed proteostasis may reflect the initiation of fibril nucleation.
What are the implications?
These findings show that αS aggregates rapidly but in two steps in the presence of N protein. This happens in a two-step process. The first step of fibril nucleation occurs quickly, leading to a plateau where the mass of fibrils is low.
These fibrils lead to the formation of, or perhaps their conversion to, another higher plateau that is more stable. This contains more fibrils and is brighter. Both these steps occur noticeably faster compared to the absence of N protein.
The N-αS binding causes charge compensation, which exposes the NAC region of the αS, which is quick to aggregate, as well as abolishing the electrostatic interactions that repel other αS molecules. The presence of other αS proteins in the complex that is prone to form aggregates may enhance nucleation and thus trigger further aggregation, as well as reducing the lag time.
The change in the FRET values indicates the effect of the N protein on αS proteostasis, with the latter protein showing a high or higher affinity for the former relative to other αS interactions.
"Our results suggest that the observed link between SARS-CoV-2 infection and Parkinson's disease might originate from a molecular interaction between virus proteins and αS." Further studies are required to confirm the long-term damage caused by the virus. Certainly, this must be ruled out before using the N protein in vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources