On March 11, 2020, the World Health Organization (WHO) declared the rapid outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to be a pandemic. To date, the coronavirus disease-19 (COVID-19), which is the disease caused by SARS-CoV-2, has infected more than 203 million and claimed the lives of over 4.3 million people worldwide. SARS-CoV-2 primarily spreads through the respiratory droplets of infected individuals and can cause mild to severe symptoms in infected individuals.
In an effort to quickly identify drugs that can be used to treat COVID-19, scientists have become increasingly interested in drug repurposing strategies. In fact, several drugs such as chloroquine, hydroxychloroquine, umifenovir, lopinavir, ribavirin, favipiravir, oseltamivir, and remdesivir, have already been tried for the treatment of COVID-19. Researchers have also suggested that rational drug designing that targets the SARS-CoV-2 protein structure could be an effective approach for the development of COVID-19 therapeutics.
 Study: A Flavonoid Based Inhibitors Targeting SARS-CoV 3CL Protease for the Treatment of SARS-CoV-2 Infection. Image Credit: Maksim Shmeljov / Shutterstock.com
Study: A Flavonoid Based Inhibitors Targeting SARS-CoV 3CL Protease for the Treatment of SARS-CoV-2 Infection. Image Credit: Maksim Shmeljov / Shutterstock.com
3CLpro of SARS-CoV-2
SARS-CoV-2 is a single-stranded and positive-sense ribonucleic acid (RNA) virus that belongs to the family Coronaviridae. SARs-CoV-2 contains two viral proteases including papain-like protease (PLpro) and 3C-like protease (3CLpro).
The 3CLpro of SARS-CoV-2, which is also known as Mpro, is an important enzyme associated with viral transcription and replication. 3CLpro is a three-domain cysteine protease, whose active binding site is present at the cleft of domains I and II. It also comprises two catalytic residues known as HIS41 and CYS145.
In its active form, 3CLpro is a homodimer that releases viral polypeptides to the host cell to ultimately aid in viral infection. Two of the main functions of 3CLpro are the maturation of viral particles and cleavage of the viral capsid.
The important role of 3CLpro in the infection by SARS-CoV-2 has therefore increased the interest of using 3CLpro as a potential target for antiviral drugs. Moreover, the ability to inhibit the activity of this viral protease could restrict viral replication.
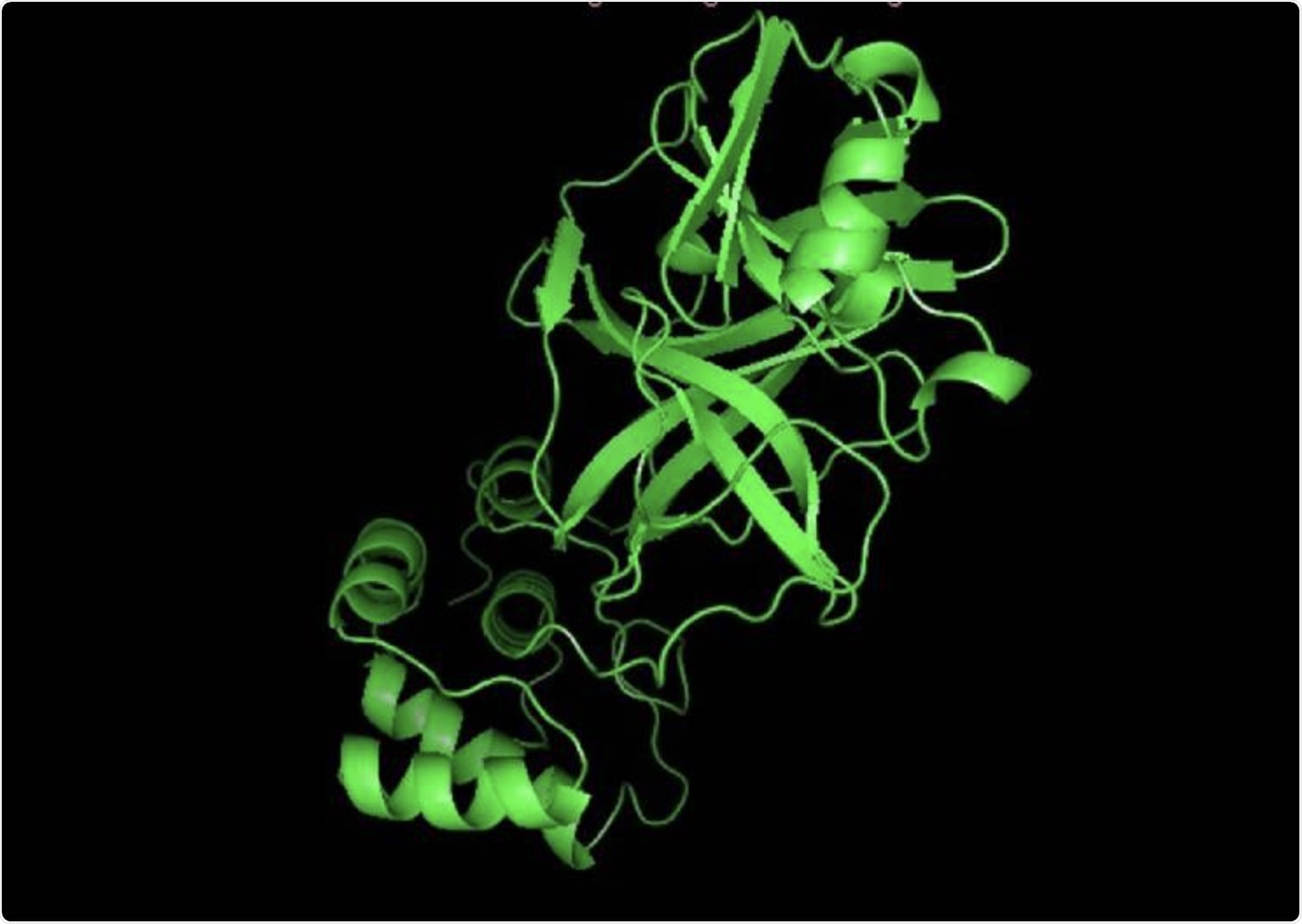 The structure of 3CL protease (6LU7).
The structure of 3CL protease (6LU7).
Flavonoids as antivirals
Previous studies have shown that flavonoids are naturally occurring polyphenolic compounds that possess antiviral properties. Quercetin is one of the common types of polyphenolic flavonoids that can inhibit proteases, reverse transcriptase, polymerases, and anti-influenza-A viruses.
An earlier in vitro study reported that quercetin can also inhibit the 3CLpro of SARS-CoV with an IC50. This report was further validated by other researchers who also found that quercetin derivatives can inhibit 3CLpro and PLpro of SARS-CoV-2. While exploring this antiviral property of flavonoids, a group of researchers revealed that these inhibitors often possess an (S)-γ -lactam and fluoro ring that occupies the S1 site of 3CLpro of SARS-CoV-2.
A new study
A new study published in the Journal of Molecular Structure has explored a series of potential flavonoid inhibitors targeting 3CL protease of SARS-CoV. In this study, a series of quercetin-based derivatives were designed by inserting a gamma lactam ring and various fluoro substituted heterocyclic ring systems to the flavonoid scaffold. Researchers used a computational approach to assess the pharmacological potential, toxicity, and drug-likeness of the designed derivatives.
In this study, researchers calculated the molecular properties and bioactivity score of the newly designed 3 CL protease inhibitors L1-L15 with the help of the molinspiration cheminformatics software. The molecular hydrophobicity was evaluated using LogP (octanol/water partition coefficient).
The Log P value indicated that the designed ligands were lipophilic and, therefore, permeable through the cell membrane. The bioactivity score obtained for several compounds indicated wide-ranging activities. Some compounds revealed considerable biological activity, while others were found to be inactive.
A solubility and toxicity assessment of the designed 3CL pro inhibitors was performed using Osiris property explorer. In this test, researchers observed good solubility in the newly designed ligands in comparison to the quercetin standard. In the toxicity test, the tested ligands showed a high risk of being tumorigenic; however, they did not exhibit mutagenic, reproductive, or other irritant toxicity profiles.
The study also analyzed the pharmacokinetic properties of the 3CL-protease inhibitors (L1-L15) including their absorption, distribution, metabolism, excretion, and toxicity (ADMET). The ADMET profile strongly supported the tested compounds as a potential drug candidate.
Aside from L1, L3, and L4, all other tested ligands were found to be non-mutagenic and non-carcinogenic. Molecular docking studies further validated the efficacy of the tested compounds as potential drugs for the treatment of COVID-19.
Conclusion
As the world continues to battle with the ongoing pandemic, the design and discovery of novel drugs remain extremely important. Rational drug design that uses computational tools has an array of benefits including high efficiency and cost-effectiveness.
The current study takes a step in this direction by proposing flavonoid-based 3CL protease inhibitors. Its findings further advocate the use of newly designed flavonoid-based 3 CL protease inhibitors for the treatment of COVID-19.