Introduction
Highly transmissible SARS-CoV-2 VOCs and the waning protection of approved coronavirus disease 2019 (COVID-19) vaccines have increased public health concerns. Breakthrough infections and cases of COVID-19-related hospitalizations have been observed in individuals vaccinated with two messenger ribonucleic acid (mRNA) doses. Therefore, a third booster vaccine is now recommended six months after full vaccination for individuals 16 years or older in most countries around the world, with those aged 12 and up approved for booster shots in the United States.
With over 7.5 million children diagnosed with COVID-19, resulting in 28,000 hospitalizations and 12,000 pediatric deaths worldwide, it is crucial to develop and implement effective vaccine regimens across the younger age groups.
About the study
In the present study, the authors aimed to test vaccine-induced antibody durability against the wildtype SARS-CoV-2 spike, RBD, and the new Omicron VOC. The researchers also quantified the antibodies produced against the wildtype SARS-CoV-2 spike, RBD, and Omicron VOC to further understand the vaccine responses.
With the consent of their parents, blood samples of the children included in the current study were collected at four time points. These time points included prior to vaccination, two to three weeks after the initial mRNA vaccination, two to four weeks after the second dose, and six months after the vaccination schedule was completed.
An in-house enzyme-linked immunosorbent assay (ELISA) was performed on the serum isolated from the blood samples to detect immunoglobulin G (IgG) against the wildtype SARS-CoV-2 spike and RBD, as well as the SARS-CoV-2 Omicron VOC RBD. The analysis was then completed using a one-way analysis of variance (ANOVA) to analyze the results.
A total of 31 children participated in the current study, out of which blood samples of 19 children were collected at the four time points. The median age of the group was 13.9 years. The cohort consisted of 27 children between the ages of 12-15 years and four children between the ages of 16-19 years.
Study findings
The durability of antibodies produced in response to the wild-type SARS-CoV-2 spike and RBD, as well as the Omicron RBD, was analyzed at each time point.
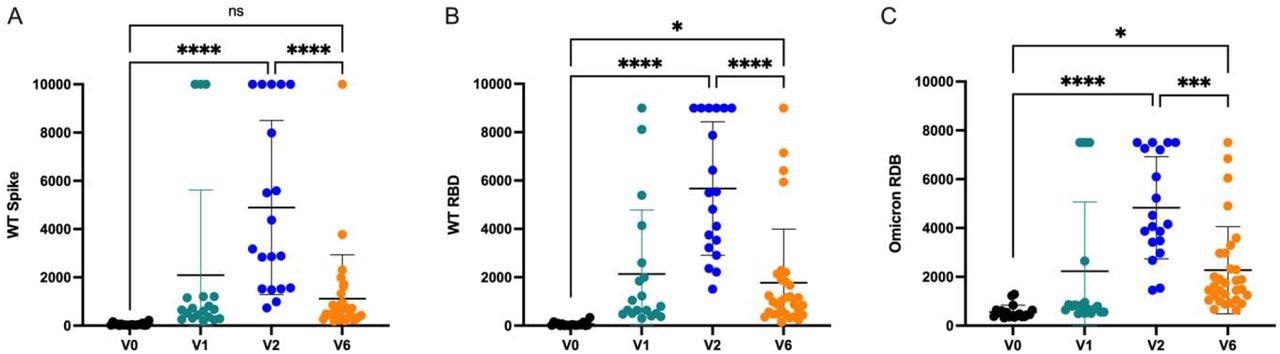
Adolescent anti-SARS-CoV-2 antibody responses over time. Humoral responses to a) Wildtype (WT) Spike b) WT Receptor Binding Domain (RBD), and c) Omicron RBD are quantified prior to vaccination, 2-3 weeks following the first vaccine dose, 2-4 weeks following the second mRNA vaccine dose, and 6 months following the second mRNA vaccine dose. Displayed as international units. Analysis by ANOVA. ns = not significant, * P < 0.05, *** P < 0.001, **** P < 0.0001
Anti-SARS-CoV-2 antibodies were abundantly generated after the second dose of the vaccine. However, a reduction in antibody production was observed six months after the second vaccination dose.
Six months following the second dose, most children exhibited levels of anti-spike or anti-RBD antibodies similar to those detected after the first dose of vaccination. This reduction in antibody response is responsible for the increased vulnerability of adolescents to breakthrough infections six months after their second vaccination dose.
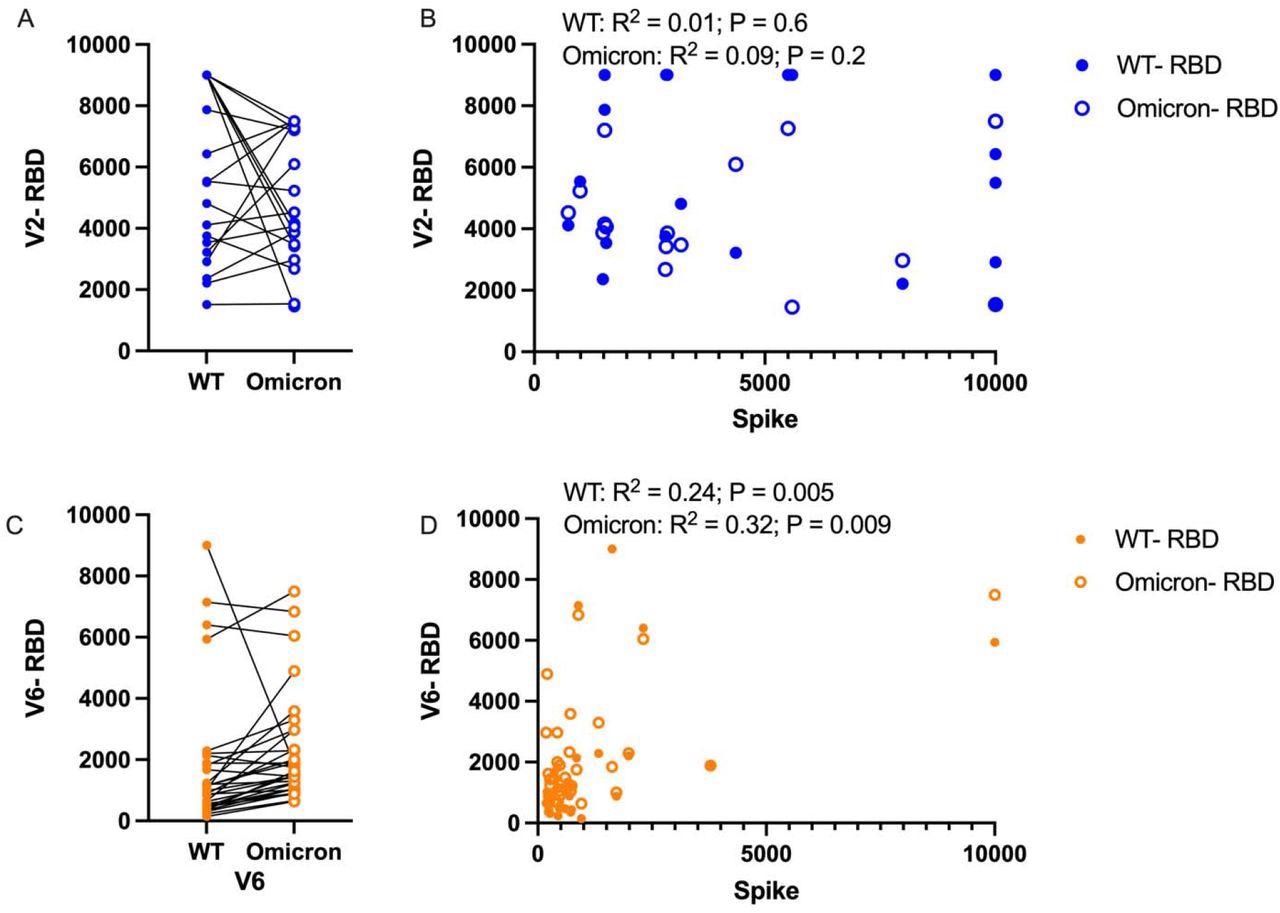
Comparison of humoral response to wild-type (WT) and Omicron RBD. A) Following the second mRNA vaccine dose, anti-RBD responses titers are compared between WT and Omicron, B) and correlations between RBD and Spike were assessed for each variant. C) Anti-RBD titers were also compared at the 6-month time point, and correlation between RBD and Spike was again assessed. Paired analysis with t-test, correlation with Pearson correlation.
A subgroup of children in the study exhibited a reduction of sensitivity towards the Omicron VOC as compared to the wildtype SARS-CoV-2 RBD after the second dose of an mRNA vaccine, while most adolescents exhibited equal sensitivity towards the Omicron RBD and wildtype SARS-CoV-2 RBD after the second vaccine.
In two to four weeks after the second vaccine dose, no correlation was observed in the peak immunity towards the wildtype SARS-CoV-2 spike and Omicron VOC. The researchers also observed a trend of increased immunity against the Omicron VOC six months after the second vaccine dose, despite the overall trend of lower total antibody titers.
Conclusions
The study results showed that mRNA vaccine-induced immunity gradually reduces to near pre-vaccination levels six months after the second vaccine dose in adolescent children. However, their immune response against the Omicron VOC was stronger than that exhibited by adults.
The authors suggest that children can develop a more adaptive humoral immune response than adults against emerging VOCs. However, the study establishes that the durability of vaccine-induced immunity gradually wanes in adolescents; therefore, the need for a booster vaccine is undeniable.
According to the authors, given the waning protection of vaccines that can be life-threatening to young adolescents, authorization for mRNA boosters for the 12-15-year-old age group can curtail the spread of SARS-CoV-2 and prevent future COVID-19-related hospitalizations.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Burns, M. D., Bartsch, Y. C., Boribong, B. P., et al. (2022). Durability and cross-reactivity of SARS-CoV-2 mRNA vaccine in adolescent children. medRxiv. doi:10.1101/2022.01.05.22268617. https://www.medrxiv.org/content/10.1101/2022.01.05.22268617v1
- Peer reviewed and published scientific report.
Burns, Madeleine D., Brittany P. Boribong, Yannic C. Bartsch, Maggie Loiselle, Kerri J. St. Denis, Maegan L. Sheehan, Jessica W. Chen, et al. 2022. “Durability and Cross-Reactivity of SARS-CoV-2 MRNA Vaccine in Adolescent Children.” Vaccines 10 (4): 492. https://doi.org/10.3390/vaccines10040492. https://www.mdpi.com/2076-393X/10/4/492.