2. What should I know before I use Intrarosa?
Do not use if you have ever had an allergic reaction to Intrarosa or any of the ingredients
listed at the end of the CMI or if you have or have had any of the conditions listed
in Section
2. What should I know before I use Intrarosa?.
Talk to your doctor before you use Intrarosa if you have any other medical conditions,
take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with Intrarosa and affect how it works.
4. How do I use Intrarosa?
One pessary once a day at bedtime.
You place it in your vagina with the provided applicator, or with your fingers. Do
not use any other applicator.
5. What should I know while using Intrarosa?
|
Things you should do
|
Remind any doctor, dentist, or pharmacist you visit that you are using Intrarosa.
Go for regular Pap tests, gynaecological and breast exams. Do this as per your healthcare
professional's directions.
If you become pregnant, stop taking Intrarosa. Talk with your healthcare professional.
|
|
Things you should not do
|
Do not stop using this medicine without telling your doctor.
Do not change the dose unless your doctor tells you to.
Do not use this medicine if you have vaginal bleeding that has not been diagnosed.
Do not use this medicine if you still have periods. This drug is for postmenopausal
women only.
|
|
Driving or using machines
|
No effects on ability to drive and use machines have been observed.
|
|
Drinking alcohol
|
There is no potential interaction with alcohol.
|
|
Looking after your medicine
|
Store Intrarosa in a cool, dry place, out of direct light where the temperature is
below 30°C. Do not freeze.
Keep Intrarosa in the original packaging, in a safe place, away from children.
|
6. Are there any side effects?
The most common side effect is vaginal discharge. A change in your breast exam or
Pap test results can occur while you take Intrarosa. Your healthcare professional
will decide when to perform breast exams and Pap tests and will interpret the results.
This medicine is subject to additional monitoring. This will allow quick identification
of new safety information. You can help by reporting any side effects you may get.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems .
Active ingredient:
prasterone
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using Intrarosa. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using Intrarosa.
Where to find information in this leaflet:
1. Why am I using Intrarosa?
Intrarosa contains the active ingredient prasterone. Prasterone belongs to a class of drugs known as steroids.
Intrarosa is used to treat vulvar and vaginal atrophy in postmenopausal women with
moderate to severe symptoms.
Prasterone is used to replace missing natural steroids in some women in order to relieve
menopausal symptoms of vulvar and vaginal atrophy such as vaginal dryness, pain during
sexual activity, irritation, or itching.
Intrarosa is a local hormone replacement therapy (HRT).
2. What should I know before I use Intrarosa?
Warnings
Do not use Intrarosa if:
You are allergic to prasterone, or any of the ingredients listed at the end of this
leaflet.
You have or have ever had breast cancer, or if you are suspected of having it;
You have cancer which is sensitive to oestrogens, such as cancer of the womb lining
(endometrium), or if you are suspected of having it;
You have excessive thickening of the womb lining (endometrial hyperplasia) that is
not being treated;
You have or have ever had a blood clot in a vein (thrombosis), such as in the legs
(deep venous thrombosis) or the lungs (pulmonary embolism);
You have a blood clotting disorder (such as protein C, protein S, or antithrombin
deficiency);
You have or recently have had a disease caused by blood clots in the arteries, such
as a heart attack, stroke or angina;
You have or have ever had a liver disease and your liver function tests have not returned
to normal;
You have a rare blood problem called “porphyria” which is passed down in families
(inherited);
You are pregnant or suspect you may be;
You are breastfeeding;
You have vaginal bleeding that has not been diagnosed.
If any of the above conditions appear for the first time while taking Intrarosa, stop
taking it at once and consult your doctor immediately.
Check with your doctor if you:
Have a vaginal infection. The infection will need to be treated with antibiotics before
starting treatment with Intrarosa.
Take any medicines for any other condition.
The use of Hormone Replacement Therapy (HRT) carries risks which need to be considered
when deciding whether to start taking it, or whether to carry on taking it.
Before you start (or restart) HRT, your doctor will ask about your own and your family's
medical history. Your doctor may decide to perform a physical examination. This may
include an examination of your breasts and/or an internal examination, if necessary.
Once you have started treatment you should see your doctor for regular check-ups.
Tell your doctor if you have ever had any of the following problems, before you start
the treatment, as these may return or become worse during treatment with Intrarosa.
If so, you should see your doctor more often for check-ups:
fibroids inside your womb;
growth of womb lining outside your womb (endometriosis) or a history of excessive
growth of the womb lining (endometrial hyperplasia);
increased risk of developing blood clots (see “Blood clots in a vein (thrombosis)”);
increased risk of getting an oestrogen-sensitive cancer (such as having a mother,
sister or grandmother who has had breast cancer);
high blood pressure;
a liver disorder, such as a benign liver tumour;
diabetes;
gallstones;
migraine or (severe) headaches;
a disease of the immune system that affects many organs of the body (systemic lupus
erythematosus, SLE);
epilepsy;
asthma;
a disease affecting the eardrum and hearing (otosclerosis);
a very high level of fat in your blood (triglycerides);
fluid retention due to cardiac or kidney problems.
HRT and cancer
Intrarosa has not been studied in women with current or history of cancers.
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer
of the lining of the womb (endometrial cancer)
Taking oestrogen-only HRT tablets for a long time can increase the risk of developing
cancer of the womb lining (the endometrium).
It is uncertain whether a risk exists with local vaginally administered products like
Intrarosa used for long-term (more than one year) treatments.
If you get bleeding or spotting, it’s usually nothing to worry about, but you should
make an appointment to see your doctor. It could be a sign that your endometrium has
become thicker.
The following risks apply to HRT medicines which circulate in the blood. It is not
known how these risks apply to locally administered treatments such as Intrarosa.
You should talk to your doctor if you are concerned.
Breast cancer
Evidence suggests that taking combined oestrogen-progestogen and possibly also oestrogen-only
HRT increases the risk of breast cancer. The risk depends on how long you are using
HRT. The additional risk becomes clear within a few years, however, it returns to
normal within a few years (at most 5) after stopping treatment.
Regularly check your breasts. See your doctor if you notice any changes such as:
dimpling of the skin;
changes in the nipple;
any lumps you can see or feel.
Additionally, you are advised to join mammography screening programs when offered
to you.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of oestrogen-only
HRT has been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. For example, in women aged 50 to 54 who
are not taking HRT, about 2 women in 2000 will be diagnosed with ovarian cancer over
a 5-year period. For women who have been taking HRT for 5 years, there will be about
3 cases per 2000 users (i.e. about 1 extra case).
Cases of ovarian and breast cancer have rarely been reported in women treated with
6.5 mg of prasterone for 52 weeks.
Effect of HRT on heart and circulation
Intrarosa has not been studied in women with history of thromboembolic diseases, uncontrolled
hypertension or heart disease.
Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3 to 3-times higher in HRT users than
in non-users, especially during the first year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain,
breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any
of the following applies to you. Inform your doctor if any of these situations apply
to you:
you are unable to walk for a long time because of major surgery, injury or illness
;
you are seriously overweight (BMI >30 kg/m2);
you have any blood clotting problem that needs long-term treatment with a medicine
used to prevent blood clots;
if any of your close relatives has ever had a blood clot in the leg, lung or another
organ;
you have systemic lupus erythematosus (SLE);
you have cancer;
you have recently had a baby.
For signs of a blood clot, see "Stop using Intrarosa and see a doctor immediately"
under Section 5.
Heart disease (heart attack) / Hypertension
For women taking oestrogen-only therapy there is no increased risk of developing a
heart disease.
Stroke
The risk of getting stroke is about 1.5 times higher in HRT users than in non-users.
The number of extra cases of stroke due to use of HRT will increase with age.
Other conditions
HRT can increase the levels of some plasma binding proteins such as thyroid binding
globulin. Hormone concentrations remain unchanged.
HRT will not prevent memory loss. There is some evidence of a higher risk of memory
loss in women who start using HRT after the age of 65. Speak to your doctor for advice;
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Intrarosa is for use in postmenopausal women only.
If you become pregnant or think you may be pregnant, stop taking Intrarosa immediately
and contact your doctor.
Intrarosa is not recommended for use during breastfeeding. It is unknown if this medicine
is excreted in human milk.
Children and adolescents
Intrarosa is only used in postmenopausal adult women.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with Intrarosa and affect how it works.
Interactions of Intrarosa with other drugs have not been established.
The use of Intrarosa in combination with HRT (oestrogen-only or oestrogen-progestogen
combination or androgen treatment) or vaginal oestrogens is not recommended.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect Intrarosa.
4. How do I use Intrarosa?
How much to take
One pessary once a day.
Follow the instructions provided and use Intrarosa until your doctor tells you to
stop.
When to take Intrarosa
Intrarosa should be used at bedtime.
How to use Intrarosa
Empty your bladder and wash and dry your hands before handling the pessary and the
applicator.
Tear off 1 pessary along the perforations from the pessary strip.
Place the pessary in your vagina with the provided applicator (A), or with your fingers
(B). Do not use any other applicator.
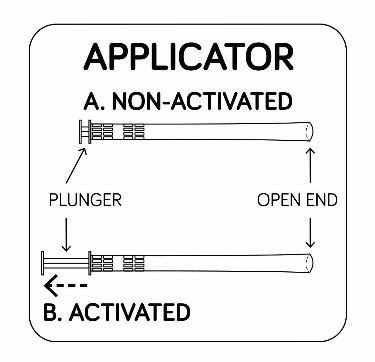
A. Using the applicator
STEP 1
1A. Remove 1 applicator from the package.
1B. The applicator must be activated before use. Pull back on the plunger until it
stops to activate the applicator.
Place the applicator on a clean surface.
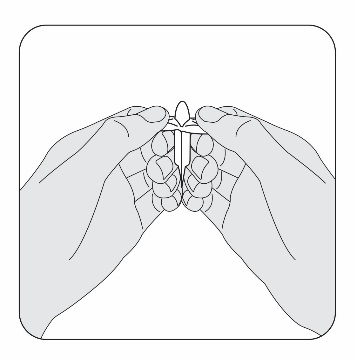
STEP 2
Slowly pull the plastic tabs on the pessary away from each other while keeping the
pessary still between your fingers. Carefully remove the pessary from the plastic
wrap. If a pessary falls on an unsanitary surface, throw it away and open a new one.
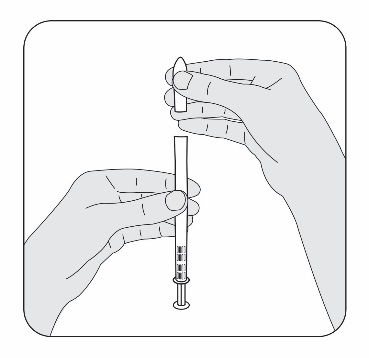
STEP 3
Place the flat end of the pessary into the open end of the activated applicator as
shown. You are now ready to insert the pessary into your vagina.
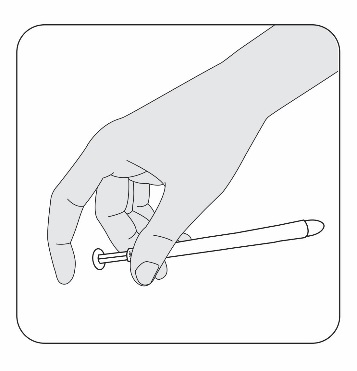
STEP 4
Hold the applicator between your thumb and middle finger. Leave your index (pointer)
finger free to press the applicator plunger after the applicator is inserted into
your vagina.
STEP 5
Select the position for insertion of the pessary that is most comfortable for you.
5a. Lying position
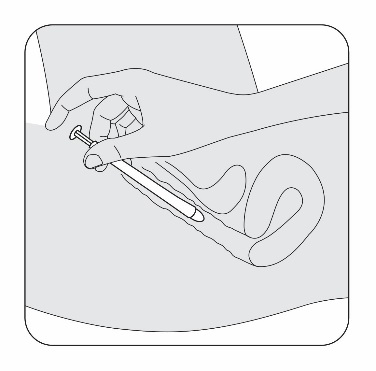
5b. Standing position
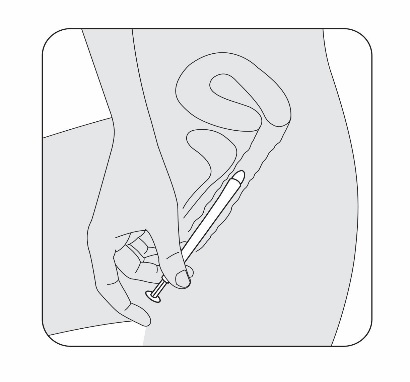
STEP 6
Gently slide the pessary end of the applicator into your vagina as far as it will
comfortably go.
Do not use force.
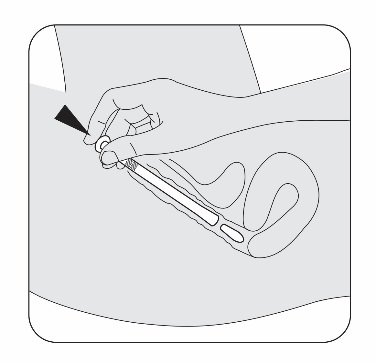
STEP 7
Press the applicator plunger with your index (pointer) finger to release the pessary.
Remove the applicator. Wash it or throw it away after using for one week (two extra
applicators are provided).
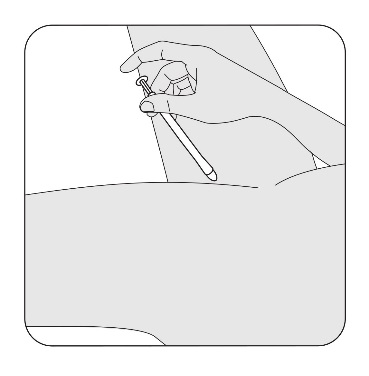
To wash the applicator:
Take the applicator apart, by pulling the plunger out of the body of the applicator
Rinse the 2 pieces for 30 seconds under running water
Wipe dry with a clean paper towel or something similar, and reassemble
Store the washed applicator in a clean place, separate to the unused applicators.
B. Using fingers
Unwrap the pessary as shown in Step 2. Place the pessary into your vagina with your
fingers as far as it can comfortably go. Do not use force.
If you forget to use Intrarosa
Intrarosa should be used regularly at the same time each day. If you miss your dose
at the usual time, use one as soon as you remember.
If your next dose is due in less than 8 hours, skip the dose you missed and take your
next dose when you are meant to. Do not use more than one pessary at a time.
Do not take a double dose to make up for the dose you missed.
If you think that you have used too much Intrarosa, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Intrarosa?
Things you should do
Once you have started using Intrarosa you should see your doctor for regular check-ups
(at least every 6 months or as clinically appropriate). At these check-ups, discuss
with your doctor the benefits and risks of continuing with Intrarosa.
Go for regular Pap tests, gynaecological and breast exams, as recommended by your
doctor.
You may have vaginal discharge due to melting of the 'hard fat base' which adds to
increased vaginal secretions due to treatment. If vaginal discharge occurs, you are
not required to stop Intrarosa.
Remind any doctor, dentist, or pharmacist you visit that you are using Intrarosa.
Things you should not do
Do not use condoms, diaphragms or cervical caps made of latex when using Intrarosa
as they may become damaged.
Stop using Intrarosa and see a doctor immediately if you:
Notice any of the following conditions when taking Intrarosa:
any of the conditions mentioned in Section 2 under 'What should I know before I use
Intrarosa';
yellowing of your skin or the whites of your eyes (jaundice). These may be signs of
a liver disease;
become pregnant;
a large rise in your blood pressure (symptoms may be headache, tiredness, dizziness);
migraine-like headaches which happen for the first time;
if you notice signs of a blood clot, such as:
- painful swelling and redness of the legs;
- sudden chest pain;
- difficulty in breathing.
Driving or using machines
Intrarosa does not affect your ability to drive or use any machines or tools.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Store below 30°C in original package to protect from light. Store it in a cool dry
place away from moisture, heat, or sunlight; for example, do not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Do not freeze.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
A change in your breast exam or Pap test results can occur while you take Intrarosa.
Your healthcare professional will decide when to perform breast exams and Pap tests
and will interpret the results.
The following diseases are reported more often in women using HRT medicines which
circulate in the blood compared to women not using HRT. These risks apply less to
vaginally administered oestrogen treatments:
breast cancer;
ovarian cancer;
blood clots in the veins of the legs or lungs (venous thromboembolism);
heart disease
stroke.
The following side effects have been reported with other HRT containing oestrogens
:
gall bladder disease;
various skin disorders;
memory loss.
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Intrarosa contains
|
Active ingredient
(main ingredient)
|
Prasterone
|
|
Other ingredients
(inactive ingredients)
|
Hard fat
|
|
Potential allergens
|
None
|
The product is supplied with an applicator made of LDPE and 1% colourant (titanium
dioxide).
Do not take this medicine if you are allergic to any of these ingredients.
What Intrarosa looks like
Intrarosa is a white to off-white, bullet-shaped pessary, approximately 28 mm long
and 9 mm in diameter at its widest end. Intrarosa comes in PVC/LDPE blister packs
of 7 pessaries each.
Intrarosa is available as a pack of 28 pessaries packaged with 6 reusable applicators.
You can reuse each applicator for up to one week (two extra applicators are provided
in case you need them).
Intrarosa may also be available as a starter pack of 7 pessaries and 1 reusable applicator.
(Aust R 391550).
Who distributes Intrarosa
Theramex Australia Pty Ltd
Level 22, 60 Margaret Street
Sydney NSW 2000
1800 THERAMEX or 1800 843 726
This leaflet was prepared in September 2023.