What are Ventricular Assist Devices?
Applications of VADs in heart failure treatment
Technological innovations driving VAD advancements
The regulatory landscape for VADs
Market trends and growth potential for VADs
Challenges and future directions in VAD development
Conclusion
References
Further reading
What are Ventricular Assist Devices?
Ventricular Assist Devices (VADs) are mechanical heart pumps.1 They use electrical and mechanical technology to support cardiac functioning by helping blood to pump around the body.1
VADs can be used as a short or long-term management strategy for heart failure and weakened heart functioning, depending on individual circumstances.2 The devices are implanted into the body, meaning open heart surgery is needed to install them.3,4
Applications of VADs in heart failure treatment
VADs are used to support those with heart failure in both acute and chronic cases. VADs can either supplement or replace heart functions by impacting one or both ventricles of the heart. The heart ventricles are chambers at the bottom of the heart which pump blood.2
Depending on which ventricles they work on, VADs are known as:
- LVADs (Left Ventricular Assist Devices)2
- RVADs (Right Ventricular Assist Devices)2
- BiVADs (Bi Ventricular Assist Devices; both ventricles)2
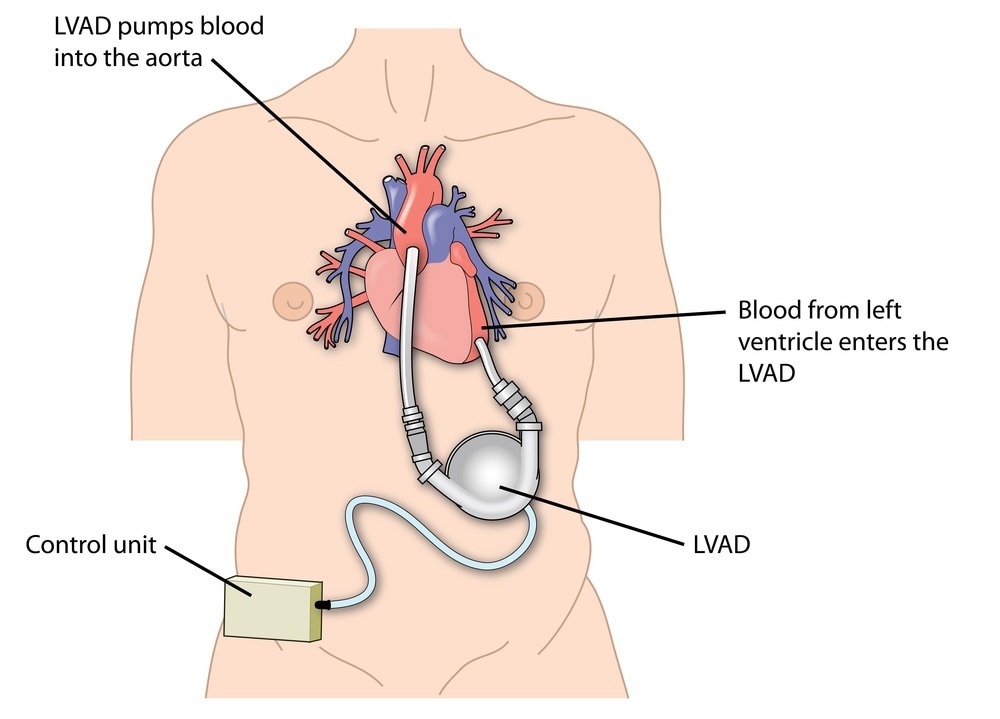 Left ventricular assist device attached to the left ventricle of the heart and the aorta. Image Credit: Blamb/Shutterstock.com
Left ventricular assist device attached to the left ventricle of the heart and the aorta. Image Credit: Blamb/Shutterstock.com
VADs can also be offered to those who are awaiting a heart transplant as a way of bridging treatment.4,5 Similarly, the devices can be a principal point of care for those who are ineligible for a heart transplant.5
Technological innovations driving VAD advancements
VAD development started in the 1960s, propelled by Domingo Liotta and the National Heart Lung and Blood Institute (NHLBI).6,7 Since then, the devices have advanced technologically to provide better support to patients and to broaden the ways that VADs may be used.4,5
Pump types
The different types of pumps used in these devices each function in different ways and have developed over time.4 Some VADs, known as pulsatile pumps, mimic how the human heart typically pumps using a continuous pulsing rhythm.8 They use positive displacement to shift fluid using mechanics.8
Other VADs use either axial flow or centrifugal pumps and are known as continuous-flow devices.1,9 Axial flow pumps work by propelling, while centrifugal pumps move fluid around by converting rotational kinetic energy to hydrodynamic energy.10 Electrical currents and magnets are used together for pump functioning.10
Left Ventricular Assist Devices
Rotor suspension in continuous-flow devices has been innovated. Newer pumps may be suspended using hydrodynamic suspension or magnetic levitation, known as “maglev,” which is a shift from the solid bearings found in older pumps.4,10
Pump size
Innovation has also led to a decrease in pump size, which is called miniaturization.3 The smaller a pump is, the smaller the incision will be for implantation, making it less invasive.3 Smaller devices being available can also offer access to a wider range of patients.11
Power supply
The power supplied to VADs is also subject to technological advancement.1 VADs are battery-powered and contain safety systems that provide backup power for when power runs low. Some types of VADs include external wearable controllers, which primarily monitor system functioning but can also provide backup power, allowing users time to charge their devices.
The regulatory landscape for VADs
As VADs are medical devices, their research and development must be approved by regulatory bodies like the Food and Drug Administration (FDA).6 VADs face particular regulatory challenges as they are invasive, meaning they have to be surgically implanted into the body or connected with other devices in the body, such as catheters.5,6 Invasive research can be difficult to approve due to the risks participants may face.6
Some types of devices may only be approved in certain areas depending on the regulatory landscape of the relevant country and where the device was tested and developed. For example, a VAD that the FDA has approved could be distributed in the US, but it would need to meet local regulations to be distributed elsewhere.
For VADs to be made available in the EU, they must have received CE marking.1,12 This certification shows they meet local safety, health, and environmental protection requirements.
Market trends and growth potential for VADs
Research suggests that the demand for VADs is growing.13 In 2020, the global VAD market size was valued at around 1.8 billion US dollars, with an expected compound annual growth rate of 17.6%.13 Some forecasts suggest that the market may reach USD 7.5 billion in 2028.13
There are several reasons why this demand may be growing. The aging population is thought to play a role, as a greater number of individuals in the population experience cardiovascular disease.4,13 Similarly, cardiovascular disease is associated with long COVID, a syndrome that impacts some after COVID-19 infection.14 As cardiovascular health may be on a downward trend, the demand for cardiac tools may increase.4,13
Challenges and future directions in VAD development
Research and development processes are fundamental to the advancement of medical technology, and VADs are no exception. Research allows VADs to be improved in terms of accessibility and innovation, as well as broadening VADs’ uses.6 However, conducting this research can be challenging.6
A relatively small, specialized population is needed to test devices, meaning that recruiting participants can be difficult.6 Moreover, the invasive nature of these devices can create difficulties in gaining ethical approval.6 The FDA is aware of these challenges and has outlined methods for clinical VAD research in the US.6
These issues may challenge the research and development cycle.6 Despite this, technological advancements are happening in the area.3 Such advancements can include alterations made to the devices available, but also to the ways that VADs are used by practitioners.3,5
In what may be a first, a patient with heart failure was successfully bridged to a heart transplant using an external maglev VAD as part of a mechanical circulatory support system.5 This was achieved by using the VAD in tandem with a catheter and drainage tube in the heart.5 This case’s success demonstrates that VAD innovation could lead to improved patient support.
Conclusion
VADs are important for managing many kinds of heart failure by supporting cardiac functioning. The importance of these devices and their development could only grow with current medical and market trends in mind.
As well as being a significant part of the MedTech sector, VADs are crucially important devices for managing cardiovascular health and heart failure for a varied group of patients. The continued development and advancement of these tools are vital to providing meaningful cardiac healthcare.
References
- Timms, D. (2011). A review of clinical ventricular assist devices. Medical Engineering & Physics, 33(9), 1041-1047. https://pubmed.ncbi.nlm.nih.gov/21665512/
- Kuroda, T., Miyagi, C., Fukamachi, K., & Karimov, J. H. (2023). Biventricular assist devices and total artificial heart: Strategies and outcomes. Frontiers in Cardiovascular Medicine, 9, 972132. https://pmc.ncbi.nlm.nih.gov/articles/PMC9853410/
- Hanke, J. S., Dogan, G., Shrestha, M., Haverich, A., & Schmitto, J. D. (2021). Innovations in implantation techniques of ventricular assist devices. JTCVS open, 8, 28-32. https://pmc.ncbi.nlm.nih.gov/articles/PMC9390346/#:~:text=Whereas%20the%20early%20versions%20of,strategies%20as%20a%20sustaining%20innovation.
- Carpenter, B. A., Gonzalez, C. J., Jessen, S. L., Moore, E. J., Thrapp, A. N., Weeks, B. R., & Clubb Jr, F. J. (2013). A brief review of ventricular assist devices and a recommended protocol for pathology evaluations. Cardiovascular Pathology, 22(5), 408-415. https://www.sciencedirect.com/science/article/abs/pii/S1054880713001002#:~:text=For%20cardiac%20devices%20in%20general,device%20(i.e.%2C%20biocompatibility).
- Li, P., Zhang, X., Chen, S., Hsu, P. L., Wu, T., Qian, S., ... & Dong, N. (2023). Case report: Successful percutaneous extracorporeal magnetic levitation ventricular assist device support in a patient with left heart failure due to dilated cardiomyopathy. Frontiers in Cardiovascular Medicine, 10, 1093794. https://pubmed.ncbi.nlm.nih.gov/36742072/
- Patel-Raman, S. M., & Chen, E. A. (2010). Past, present, and future regulatory aspects of ventricular assist devices. Journal of Cardiovascular Translational Research, 3, 600-603. https://pubmed.ncbi.nlm.nih.gov/21046301/
- Liotta, D. (2002). Early clinical application of assisted circulation. Texas Heart Institute Journal, 29(3), 229. https://pmc.ncbi.nlm.nih.gov/articles/PMC124772/
- Schulman, A. R., Martens, T. P., Christos, P. J., Russo, M. J., Comas, G. M., Cheema, F. H., ... & Naka, Y. (2007). Comparisons of infection complications between continuous flow and pulsatile flow left ventricular assist devices. The Journal of Thoracic and Cardiovascular Surgery, 133(3), 841-842. https://www.jtcvs.org/article/S0022-5223(06)02102-7/fulltext
- Slaughter, M. S., Pagani, F. D., Rogers, J. G., Miller, L. W., Sun, B., Russell, S. D., ... & HeartMate II Clinical Investigators. (2010). Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. The Journal of Heart and Lung Transplantation, 29(4), S1-S39. https://pubmed.ncbi.nlm.nih.gov/20510628/
- Miyamoto, T., Fukamachi, K., & Karimov, J. H. (2022). Continuous-Flow Ventricular Assist Devices. In Advances in Cardiovascular Technology (pp. 79-119). Academic Press.
- Adachi, I., Burki, S., Zafar, F., & Morales, D. L. S. (2015). Pediatric ventricular assist devices. Journal of Thoracic Disease, 7(12), 2194. https://jtd.amegroups.org/article/view/6182/html
- French-Mowat, E., & Burnett, J. (2012). How are medical devices regulated in the European Union? Journal of the Royal Society of Medicine, 105(1_suppl), 22-28. https://pmc.ncbi.nlm.nih.gov/articles/PMC3326593/
- Grand View Research. (2021). Ventricular Assist Device Market Size, Share & Trends Analysis Report By Product, By Type Of Flow (Pulsatile Flow, Continuous Flow), By Application, By Design, By Region, And Segment Forecasts, 2021 - 2028. 978-1-68038-603-5. https://www.grandviewresearch.com/industry-analysis/ventricular-assist-devices-market
- Tsampasian, V., Bäck, M., Bernardi, M., Cavarretta, E., Dębski, M., Gati, S., ... & Vassiliou, V. S. (2024). Cardiovascular disease as part of Long COVID: a systematic review. European Journal of Preventive Cardiology, zwae070. https://pubmed.ncbi.nlm.nih.gov/38381595/
Further Reading
Last Updated: Oct 18, 2024