Acute gastroenteritis caused by infection with rotavirus represents a serious global issue associated with significant morbidity and mortality among infants and young children. It has recently been estimated that more than half a million rotavirus-related deaths occur each year among children younger than 5 years of age.
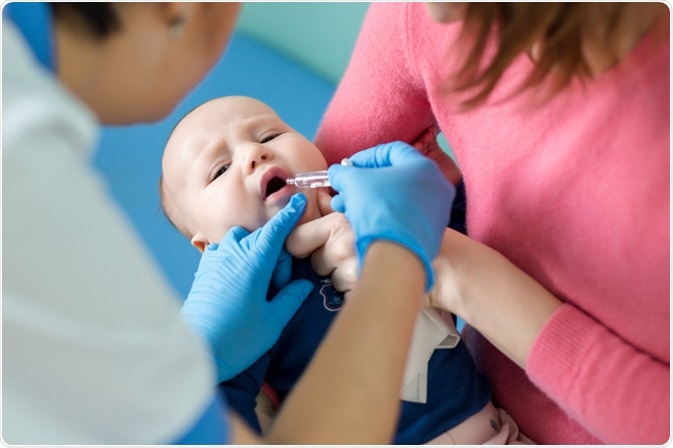
Image Credit: Gorlov-KV / Shutterstock.com
Despite the availability of oral rehydration solutions and improved sanitation measures, rotavirus continues to be a threat. Clinical and population-based studies have shown that the new rotavirus vaccines are safe and effective in preventing severe rotavirus-associated diarrhea and mortality, and also reduce the impact on public health resources.
Hygiene improvements and disease tracking
Sanitation, in general, had a tremendous effect on diarrheal disease due to bacteria and parasites, but less of an impact on rotaviral disease. This is evinced by the persistence of rotavirus in high-income countries and the likely transmission of the pathogen through person-to-person contact, which persists even as fecal-oral transmission subsides. Furthermore, improvement in sanitation did not reduce the prevalence of hospital admission.
Meta-analyses have shown that improved hand hygiene reduced the total burden of gastrointestinal illness by 31%. The usage of regular soap has shown to be most beneficial, while antibacterial soap provided little additional benefit. Immediate disposal of soiled diapers in sealed containers is also of the uttermost importance.
In 2008, the World Health Organization (WHO) linked existing regional surveillance networks to establish a standardized global sentinel hospital surveillance network for rotavirus disease. This network encompasses sentinel surveillance hospitals and laboratories that report clinical features, as well as rotavirus testing data and hospitalizations, of children younger than 5 years of age to ministries of health and WHO.
Vaccination
Vaccination remains the primary public health intervention for tackling the rotaviral disease. The United States Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices recommends routine vaccination with an oral live vaccine at two, four, and six months of age.
The first rotavirus vaccines were based on monovalent rotavirus strains isolated from either rhesus or bovine hosts; nevertheless, their development was abandoned due to variable efficacy in clinical trials. Subsequently, multivalent animal-human reassortant rotavirus vaccines were produced using gene reassortment.
In 1998, a rhesus-based tetravalent rotavirus vaccine known as RRV-TV or Rotashield was recommended for routine vaccination of infants in the United States. However, the vaccine was withdrawn from the market within one year of its introduction because of its association with intussusception, which is a condition in which one segment of the intestine “telescopes” inside of another, causing an intestinal obstruction.
In 2006, two new oral live-attenuated rotavirus vaccines including Rotarix (GlaxoSmithKline, Rixensart, Belgium) and RotaTeq (Merck and Co, Sanofi Pasteur MSD, Lyon, France) became available for use. Both of these vaccines have shown high efficacy and strong safety records based on extensive studies, including randomized clinical trials.
Rotarix (RV1)
Rotarix, which is administered as a two-dose schedule, represents a monovalent human vaccine originating from a G1P[8] strain. Conversely, RotaTeq is administered as a three-dose schedule and contains five human-bovine reassortant strains (G1, G2, G3, G4, and P1A[8]); hence it is considered a pentavalent vaccine.
In April 2009, the WHO Strategic Advisory Group of Experts (SAGE) recommended that all national immunization programs should include rotavirus vaccination for infants. The incidence of rotavirus disease has dropped markedly in all the countries that introduced the vaccine.
Due to possible safety issues associated with the use of Rotarix and RotaTeq, several third-generation rotavirus vaccines are currently in development. Parenteral immunization with the inactive virus has been effective in animal models; however, to date, no proof of principle for this approach exists for human beings.
In conclusion, rotaviral gastroenteritis is considered to be a vaccine-preventable illness, based on successful outcomes in children and a drop in hospitalizations in developed countries. Despite this impressive progress, much remains to be learned about rotavirus infection, as we still do not understand the mechanism by which current vaccines induce immunity to a myriad of serotypes found in nature.
References
Further Reading
Last Updated: Dec 1, 2022