This article and associated images are based on a poster originally authored by Steven D. Broadbent, Sian Humphrey, Signe Springe, Sung Min Yang, Ayan Ghoshal, Nina Dedic and Ashley Barnes and presented at ELRIG Drug Discovery 2024 in affiliation with Axol Bioscience Ltd. and Sumitomo Pharma America, Inc.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
Neurodegenerative diseases (NDDs) such as Alzheimer's Disease and Parkinson's Disease are a leading cause of physical and cognitive disability, affecting around 15% of the global population.1 With rising prevalence due to an aging population, there is a growing need for better, safer therapies and curative treatments. The cortex is a key area affected by NDD and is also a common site of drug-induced neurotoxicity (a leading cause of drug attrition); hence, it is a major target for drug discovery.2 However, the lack of physiologically relevant cortical models is a major challenge, with traditional animal models failing to translate from "bench to clinic" and the low complexity afforded by simple cell culture and cell line models.
In partnership with Sumitomo Pharma America, Inc., Axol Bioscience performed a blinded study testing eight reference compounds on an isogenic cortical tri-culture axoModel, using an Axion multi-electrode array (MEA) system to measure electrophysiological response. Here, we demonstrate robust identification of the reference compound's mode of action, validating this axoModel for use in human-relevant drug discovery and neurotoxicity screening.
Methods
axoCells™ isogenic human iPSC-derived cortical excitatory neurons [CENs, ax0015], cortical inhibitory interneurons [CINs, ax0667], and astrocytes [ax0665] were co-cultured on 48-well Axion Maestro MEA plates according to Axol’s user guide. Cells were cultured from thaw for 26 days before compound treatment to allow full neuronal maturation and network formation.
On Day 26, recordings were taken 10 minutes pre-compound addition (baseline data, R1), 10 minutes after compound addition (R2), and 60 minutes after compound addition (longer-term effects, R3). This process was repeated twice, with a second and third dose added accumulatively.
Three concentrations of each compound were tested, and the precise concentrations were specific to each. All compounds had been blinded before shipment and were only unblinded after the final data analysis and written report. For the drug additions, 30 μL for the cell culture medium was removed and replaced with 30 μL medium containing 10x concentrated compound, resulting in the desired final compound concentration. All compounds and concentrations were tested at n=3-14 wells per compound.
The Axion Maestro MEA system was used to record 18 electrophysiological parameters, including individual electrode spike parameters, electrode burst parameters, well activity parameters, and overall network parameters. To add comparison, all parameters for each well were normalized to their R1 values. This enabled the classification of each blinded compound into one of six groups and sub-groups based on their observed actions:
Group 1 (“Little Effect”) showed minimal significantly different effects from the vehicle control. Group 2 (“Activators”) induced an overall increase in activity. This was further sub-divided into Group 2A (“Hyperpolarizing”: increased spike rate, decreased bursting), Group 2B –(“Excitatory”: increased spike rate, unchanged bursting), and Group 2C (“Pro-convulsant”: increased spike rate and bursting, typical of pro-convulsant compounds).
Group 3 (“De-Activators”) produced an overall decrease in activity. This was subdivided into Group 3A (“Blockers”: reduced neuronal activity and spike amplitude) and Group 3B (“Inhibitors”: decreased activity with unchanged spike amplitude).
After the compounds were grouped and the final report submitted, they were unblinded, and the results were compared with their known pharmacology.
axoCells iPSC-derived cells demonstrate key marker expression on immunocytochemistry (ICC)
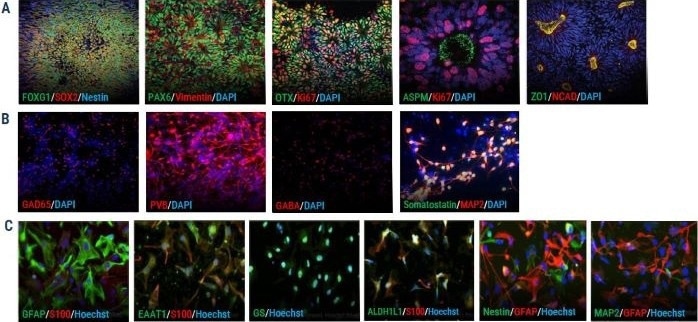
Figure 1. ICC shows expression of key markers for axoCells cortical excitatory neurons (CENs), cortical inhibitory interneurons (CINs) and astrocytes. (A) axoCells iPSC-derived neural stem cells (NSCs, ax0015) show expression of all standard NSC markers: FOXG1, SOX2, PAX6, Vimentin, OTX, Ki67, ZO1 and NCAD. (B) In contrast, axoCells CINs (DIV20, ax0667) express the key markers GAD65, Parvalbumin (PVB), Beta, Somatastatin and MAP2. (C) axoCells iPSCderived astrocytes (ax0665) express key astrocyte-specific markers (GFAP and S100B) and astrocyte-associated markers, EAAT1, Glutamine Synthetase (GS) and ALDH1L1, while showing very low levels of neuronal progenitor markers Nestin and MAP2. Image Credit: Image courtesy of Steven D. Broadbent et al., in partnership with ELRIG (UK) Ltd.
Modulation of cortical neuron firing in co- and tri-culture
Monoculture: axoCells cortical excitatory neurons
Co-culture: axoCells cortical neurons and cortical inhibitory interneurons
Tri-culture: axoCells cortical neurons, cortical inhibitory interneurons & astrocytes
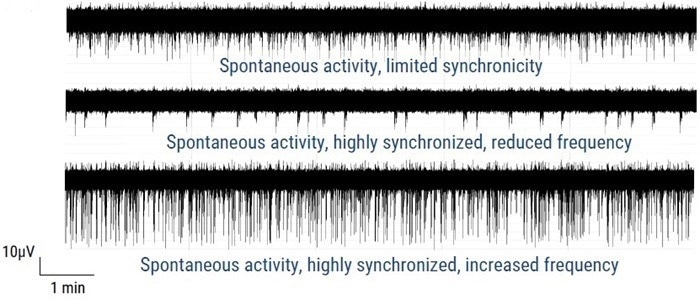
Figure 2. Axion MEA traces of axoCells CENs in monoculture, in isogenic co-culture with axoCells CINs and in isogenic tri-culture with axoCells CINs & astrocytes. The Axion MEA traces above show typical activities on DIV35. In monoculture (Top) the CENs fire frequently and spontaneously but with limited synchronicity. The addition of CINs (Middle) markedly reduces firing rate but switches activity to a more regular synchronized burst firing pattern. The addition of astrocytes (Bottom) increases both firing rate and spike amplitude while maintaining the regular synchronized burst firing seen in the co-culture model. This shows the expected functional modulation with increasing model complexity, demonstrating functional validation of the model. Image Credit: Image courtesy of Steven D. Broadbent et al., in partnership with ELRIG (UK) Ltd.
Blinded in vitro compound screening using electrophysiological endpoints
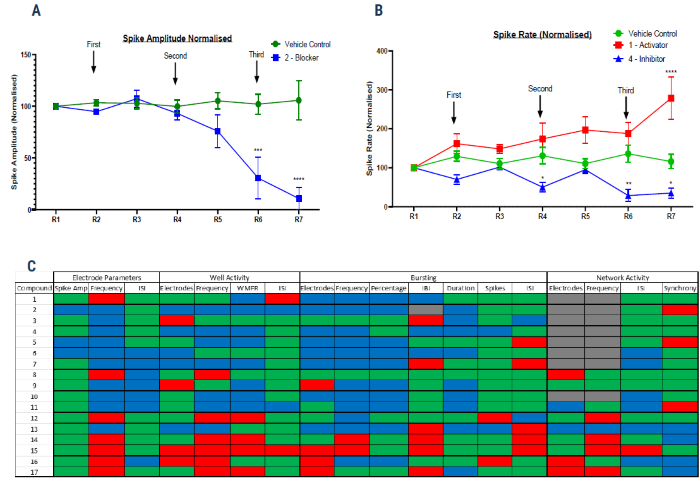
Figure 3. Blinded compound screening on a cortical tri-culture axoModel, with electrophysiological endpoints measured on the Axion Maestro MEA system. For more details on Axol Bioscience’s tri-culture protocol timeline, please see the QR code at the bottom. (A) Example spike amplitude data for vehicle control and Compound 2 (a “Blocker”) (B) Example spike rate data for vehicle control, Compound 1 (a “Hyperpolarizing Activator”) and Compound 4 (an “Inhibitor”). Statistical significance was assessed by two-way ANOVA. (C) Heatmap of the monitored parameters on the Axion MEA for the blinded compound list. Seventeen compounds were tested in total, with nine novel compounds of undefined action, two anonymized proprietary compounds, designated Compound A and Compound B, and six compounds of known action. Each parameter was color-coded GREEN for Unchanged, RED for Increased and BLUE for Decreased compared to the vehicle control. Image Credit: Image courtesy of Steven D. Broadbent et al., in partnership with ELRIG (UK) Ltd.
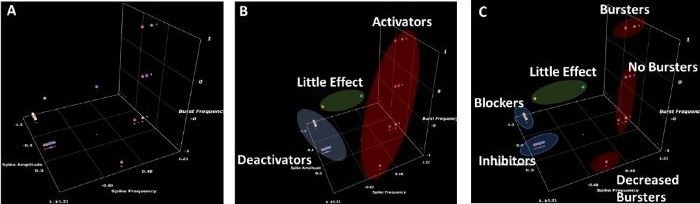
Figure 4. 3D scatter plots of measured MEA parameters for blinded compounds and vehicle control. (A) Unclustered data (B) Data clustered by highest level groupings (Little Effect, Deactivators and Activators) (C) Data clustered by sub-groupings (Little Effect, Blockers, Inhibitors, Increased Bursters, Unchanged Bursters and Decreased Bursters). Increased Bursters were identified as potentially proconvulsant, Unchanged Bursters are potentially non pro-convulsant and Decreased Bursters are hyperpolarizing. Created using the software available at https://miabellaai.net/. Image Credit: Image courtesy of Steven D. Broadbent et al., in partnership with ELRIG (UK) Ltd.
Unblinding of compounds
| Compound |
Group |
Subgroup |
| Number |
Name |
Model Classification |
Unblinded/
literature
classification |
Model Classification |
Unblinded/
literature
classification |
| 1 |
NS 309 |
Activator |
Activator |
Activator – Decreased Bursting |
Activator – Decreased Bursting |
| 2 |
Compound A |
De-activator |
De-activator |
De-activator- Blocker |
De-activator - Inhibitor |
| 3 |
Nifedipine |
Little Effect |
Little Effect |
Little Effect |
Little Effect |
| 4 |
Compound B |
De-activator |
De-activator |
De-activator- Inhibitor |
De-activator- Inhibitor |
| 5 |
Lamotrigine |
De-activator |
De-activator |
De-activator- Blocker |
De-activator- Blocker |
| 14 |
4-AP |
Activator |
Activator |
Activator – Increased Bursting |
Activator – Increased Bursting |
| 16 |
Levetiracetam |
Activator |
Activator |
Activator – Unchanged Bursting |
Activator – Unchanged Bursting |
| 17 |
Lithium Carbonate |
Activator |
Activator |
Activator – Unchanged Bursting |
Activator – Unchanged Bursting |
Figure 5. Table of unblinded compounds showing almost perfect fidelity between model-based predicted action and actual action. After unblinding, the actual reported action of the compound was compared to the predicted effect using the Axion Maestro MEA system. The results demonstrated the alignment of the predicted and actual Group in 8 out of 8 compounds, with the correct prediction of the Subgroup in 7 out of 8 compounds. The client deemed these results to be a valuable neurological compound screening test system with future drug discovery and neurotoxicity screening applications. Image Credit: Image courtesy of Steven D. Broadbent et al., in partnership with ELRIG (UK) Ltd.
Conclusion
In this blinded study, a human iPSC-derived cortical tri-culture axoModel was used to correctly identify the effect of 8 compounds, aligning 8 out of 8 Groups and 7 out of 8 Subgroups. By incorporating human iPSC-derived cells into advanced in vitro platforms, it is possible to produce human-relevant test models that could better recapitulate the complex in vivo interactions of the human cortex compared to the equivalent heterologous cell lines and animal models.
Although these experiments were carried out using cells derived from a single healthy control donor, it is possible to incorporate lines derived from multiple donors and patients with NDDs, such as Alzheimer’s Disease, to add further complexity and build disease-specific in vitro models for drug development.
References
- Feigin, V.L., Vos, T., et al. (2020). The global burden of neurological disorders: translating evidence into policy. The Lancet Neurology, [online] 19(3), pp.255–265. https://doi.org/10.1016/s1474-4422(19)30411-9.
- Weaver, R.J. and Valentin, J.-P. (2019). Today’s Challenges to De-Risk and Predict Drug Safety in Human ‘Mind-the-Gap’. Toxicological Sciences, [online] 167(2), pp.307–321. https://doi.org/10.1093/toxsci/kfy270.
About AXOL Biosciences
Axol specializes in human cell culture.
Axol produces high quality human cell products and critical reagents such as media and growth supplements. We have a passion for great science, delivering epic support and innovating future products to help our customers advance faster in their research.
Our expertise includes reprogramming cells to iPSCs and then differentiating to various cell types. We supply differentiated cells derived from healthy donors and patients of specific disease backgrounds. As a service, we also take cells provided by customers (primary or iPSC) and then do the reprogramming (when necessary) and differentiation. Clearly, by offloading the burden of generating cells, your time is freed up to focus on the research. Axol holds the necessary licenses that are required to do iPSC work.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 18, 2024