In Alzheimer’s and other diseases with complex genetics, differentiating a single induced pluripotent stem cell (iPSC) line from a single endpoint cell may improperly represent the underlying diversity.1
Cohort-level models, where several donor stem cells are converted into different end-cell types, can be utilized for in vitro models that address such challenges.
In previous work, Axol Bioscience discussed how it is possible to generate iPSC-derived neurons and neuroinflammatory cells and use them in complex co-culture models to screen compounds and develop drugs.2
In the following article, the development of differentiation processes for several axoLinesTM iPSC lines at the same time will be discussed.
Axol Bioscience developed eight iPSC lines: two control lines and six from people with Alzheimer’s Disease, with mutations in presenilin 1, homozygous ApoE4, or heterozygous ApoE4/ApoE3 genotype. Subsequently, the ApoE genotype of PSEN mutant lines was identified.
By increasing the efficiency of the differentiation of axoCellsTM astrocytes, cortical excitatory neurons, and microglia, researchers can improve how they regulate the quality and consistency of cells utilized in sophisticated in vitro models, resulting in better therapeutics for challenging neurodegenerative conditions.
Methods
Axol Bioscience differentiated its axons iPSCs into axoCellsastrocytes, cortical excitatory neurons, and microglia. As a part of their quality control (QC) processes, they next assessed the expression of crucial pluripotency and endpoint cell markers for each cell type via flow cytometry. The resulting iPSC lines (Table 1) and QC procedures (Table 2) are as follows:
Table 1. Patient-derived axoLines that were differentiated to axoCells cortical excitatory neurons, astrocytes and microglia. Source: Axol Bioscience Ltd
| iPSC line |
Status at time of sampling |
Gender |
Age |
ApoE |
Variant |
| ax7015 |
Healthy |
Male |
Newborn |
- |
- |
| ax7111 |
AD |
Female |
87 |
ApoE4/E4 |
- |
| ax7112 |
AD |
Female |
38 |
ApoE3/E3 |
PSEN-1 L286V |
| ax7113 |
AD |
Male |
53 |
ApoE2/E3 |
PSEN-1 M146L |
| ax7114 |
AD |
Female |
31 |
ApoE3/E4 |
PSEN-1 A246E |
| CENSOi074-A |
AD |
Male |
60 |
ApoE3/4 |
- |
| CENSOi077-C |
AD |
Female |
52 |
ApoE3/4 |
- |
| CENSOi004-E |
Healthy |
Male |
40-50 |
- |
- |
Table 2. Quality control parameters for axoCells astrocytes, cortical excitatory neurons and microglia. Source: Axol Bioscience Ltd
| Quality Control Test |
Method |
Details |
| iPSC |
Microbiology/ Microscopy/ Cell count
Flow cytometry
G-banding/ qPCR |
Sterility/mycoplasma free/ correct morphology and viability
Pluripotency markers: SSEA4, OCT3/4 & TRA-1-60 >70%,
Differentiation marker: SSEA1< 10%
Normal karyotype/ Sendai clearance from reprogramming |
| Cortical neuron QC |
Immunocytochemistry |
Presence of BRN2 and CUX1, TUJ1, MAP2
Absence of GFAP and OLIG2 |
| Astrocyte QC |
Immunocytochemistry |
Presence of GFAP, Nestin, EAAT2, S100ββ, ALDH1, AQP4, TUJ1 |
| Microglial QC |
Immunocytochemistry |
Presence of TMEM119, Iba1, P2RY12, CX3CR1 |
Results
axoCellsTM astrocytes
While differentiating astrocytes for this research, Axol Bioscience found that each iPSC line responded differently. Thus, Axol Bioscience utilized continuous observation of morphology to detect suboptimal differentiation earlier in the procedure and make any necessary changes (Fig. 2).
To regulate the workflow for optimization purposes, Axol Bioscience modified passaging ratios and maturation times (Fig. 2). As a result, astrocyte precursors are now regularly cryopreserved to facilitate rapid repeats from later stages where parameters are altered.
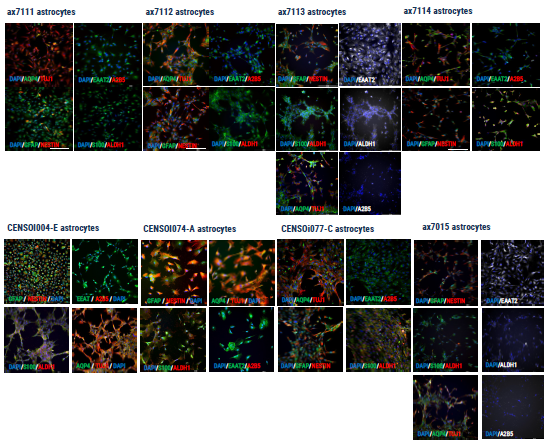
Figure 1. Immunocytochemistry demonstrating the expression of key astrocyte markers. Image Credit: Axol Bioscience Ltd
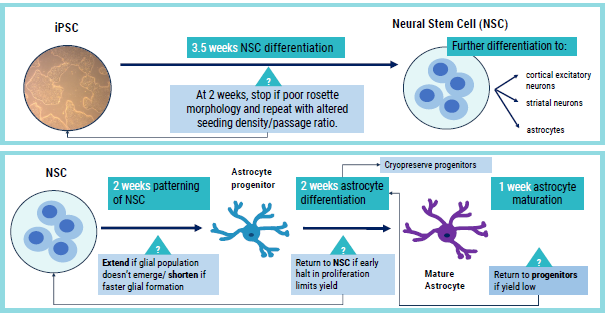
Figure 2. Schematic of axoCellsastrocyte differentiation with intervention points for optimization. Image Credit: Axol Bioscience Ltd
axoCells cortical excitatory neurons
Axol Bioscience is initiating routine differentiation of neural stem cells (NSCs) to axoCells cortical excitatory neurons or striatal neurons for quality control purposes.
They found that all cortical neuron lines reported negative for expression of oligodendrocyte marker OLIG1 and astrocyte marker GFAP (data not shown). Meanwhile, expression of markers such as BRN2 (POU3F2) are linked to more superficial layers of the cortex, suggesting heightened maturity.
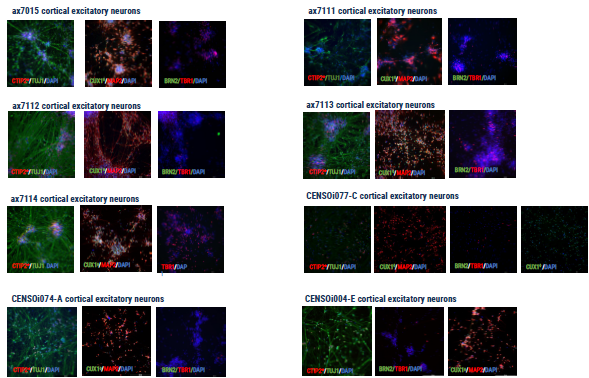
Figure 3. Immunocytochemistry demonstrating the expression of key cortical neuronal markers. Image Credit: Axol Bioscience Ltd
*CTIP2 also known as BCL11B
◊CUX1 also known as CASP
axoCells microglia
Axol Bioscience discovered that the first microglia yield differed among cell lines. Yield can eventually be increased to correspond with the growing number and size of flasks.
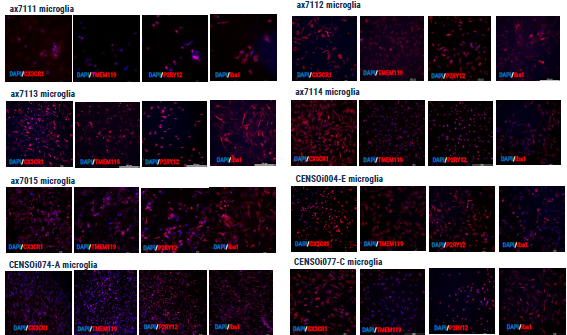
Figure 4. Immunocytochemistry demonstrating the expression of key microglial markers. Image Credit: Axol Bioscience Ltd
Conclusion
Differentiating axoLines iPSC lines to axoCells astrocytes, cortical excitatory neurons, and microglia enabled Axol Bioscience to distinguish critical steps for optimization and standardizing their processes. This standardization is essential for producing high-quality neurons and neuroinflammatory cells in in vitro disease models relevant to humans.
Further research will include functional assays, such as phagocytosis and cytokine release in microglia and spontaneous firing of cortical excitatory neurons in triculture, to advance the characterization of these lines and improve understanding of phenotypic differences.
Download the Poster Version
References and further reading
- Penney, J., Ralvenius, W.T. & Tsai, LH. Modelling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry 25, 148–167 (2020). https://doi.org/10.1038/s41380-019-0468-3
- Steven D. Broadbent, Sian Humphrey, Signe Springe, Sung Min Yang, Ayan Ghoshal, Nina Dedic & Ashley Barnes. Validation of a cortical tri-culture axoModel for in vitro compound screening a blinded compound study. Poster PSTR389.08, Neuroscience 2023
About AXOL Bioscience
The first choice for high-quality, functionally relevant iPSC-derived cells.
With over a decade of experience, we’ve developed the manufacturing capabilities to produce high-quality, functional iPSC-derived cells with excellent consistency.
Your research can benefit from our quality-focused approach, with our catalog of robust, highly relevant iPSC-derived neurons and cardiomyocytes developed at our ISO 9001:2015-accredited production facility.
Our leading neuronal cell types include: cortical excitatory neurons, striatal neurons, cortical inhibitory interneurons, microglia, astrocytes, sensory neurons and motor neurons. We also provide high-quality atrial cardiomyocytes and ventricular cardiomyocytes, as well as made-to-order myotubes.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.