Neurodegenerative diseases (NDDs), including Alzheimer's Disease and Parkinson's Disease, are among the most common causes of physical and cognitive disability, experienced by approximately 15% of the population worldwide.1 Their prevalence is expected to increase as the world’s population ages, meaning there is an increasing need for more effective, safer therapies and curative options.
The cortex is a critical area impacted by NDD. It is a common site of drug-induced neurotoxicity (a leading cause of drug attrition), making it a significant target for drug discovery efforts.2 A dearth of physiologically relevant cortical models presents a significant roadblock, with standard animal models unable to translate to humans and low complexity from simple cell culture and cell line models.
In partnership with Sumitomo Pharma America, Inc., Axol Bioscience conducted a blinded study investigating eight reference compounds on an isogenic cortical tri-culture axoModel, via an Axion multi-electrode array (MEA) system to assess electrophysiological response. In the following study, researchers show solid identification of reference compound modes of action, verifying this axoModel for application in discovering drugs for humans and screening for neurotoxicity.
Methods
axoCellsTM isogenic human iPSC-derived cortical excitatory neurons [CENs, ax0015], cortical inhibitory interneurons [CINs, ax0667], and astrocytes [ax0665] were cultured together on 48-well Axion Maestro MEA plates as per Axol’s user instructions. Cells were cultured from thaw for 26 days before treatment with the compound to enable full maturation of neurons and formation of networks
On Day 26, recordings were collected 10 minutes before adding the compound (baseline data, R1), 10 minutes following compound addition (R2), and 60 minutes following compound addition (longer-term effects, R3). This procedure was conducted twice, gradually adding a second and third dose. Altogether, three concentrations of each compound underwent testing, with precise concentrations specific to each.
All compounds were blinded before being shipped and were unblinded following the final data analysis and corresponding written report. Concerning drug additions, 30 μL of the cell culture medium was removed and substituted with 30 μL medium carrying 10x the concentrated compound, bringing about the sought-after final compound concentration. Tests of all compounds and concentrations were conducted at n=3-14 wells per compound.
The Axion Maestro MEA system was utilized to take note of 18 electrophysiological parameters. These included individual electrode spike parameters, electrode burst parameters, well activity parameters, and overall network parameters. For comparison, all parameters for the different wells were normalized to their R1 values. This permitted the classification of each blinded compound into six groups and sub-groups according to their observed behavior:
- Group 1 (“Little Effect”) displayed minimal significantly different effects compared to the vehicle control.
- Group 2 (“Activators”) produced a general increase in activity. This was later sub-divided into:
- Group 2A - (“Hyperpolarizing”: heightened spike rate, reduced bursting)
- Group 2B –(“Excitatory”: heightened spike rate, unaltered bursting)
- Group 2C - (“Pro-convulsant”: heightened spike rate and bursting, expected of pro-convulsant compounds)
- Group 3 (“De-Activators”) induced a general decline in activity. This was then subdivided into:
- Group 3A (“Blockers”: decreased neuronal activity and spike amplitude) and
- Group 3B (“Inhibitors”: reduced activity with unaltered spike amplitude).
After the compounds were arranged in categories and the final report set forth, they were unblinded, and the outcomes were evaluated in comparison with their known pharmacologies.
axoCells iPSC-derived cells demonstrate key marker expression on immunocytochemistry (ICC)
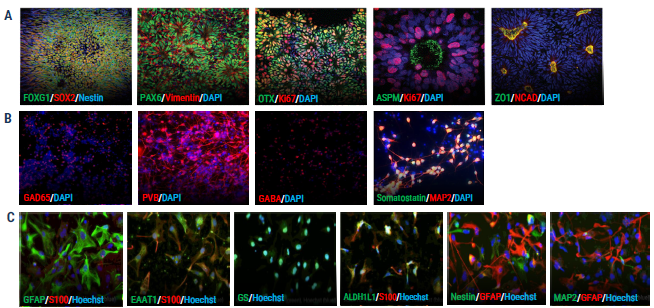
Figure 1. ICC shows expression of key markers for axoCells cortical excitatory neurons (CENs), cortical inhibitory interneurons (CINs) and astrocytes. (A) axoCells iPSC-derived neural stem cells (NSCs, ax0015) show expression of all standard NSC markers: FOXG1, SOX2, PAX6, Vimentin, OTX, Ki67, ZO1 and NCAD. (B) In contrast, axoCells CINs (DIV20, ax0667) express the key markers GAD65, Parvalbumin (PVB), Beta, Somatastatin and MAP2. (C) axoCells iPSCderived astrocytes (ax0665) express key astrocyte-specific markers (GFAP and S100B) and astrocyte-associated markers, EAAT1, Glutamine Synthetase (GS) and ALDH1L1, while showing very low levels of neuronal progenitor markers Nestin and MAP2. Image Credit: Axol Bioscience Ltd
Modulation of cortical neuron firing in co- and tri-culture
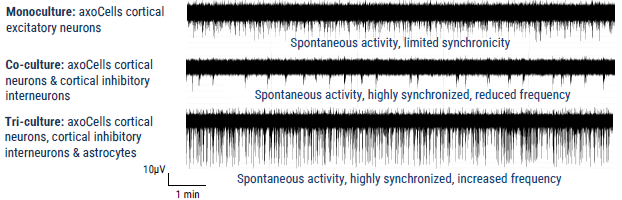
Figure 2. Axion MEA traces of axoCells CENs in monoculture, in isogenic co-culture with axoCells CINs and in isogenic tri-culture with axoCells CINs & astrocytes. The Axion MEA traces above show typical activities on DIV35. In monoculture (Top) the CENs fire frequently and spontaneously but with limited synchronicity. The addition of CINs (Middle) markedly reduces firing rate but switches activity to a more regular synchronized burst firing pattern. The addition of astrocytes (Bottom) increases both firing rate and spike amplitude while maintaining the regular synchronized burst firing seen in the co-culture model. This shows the expected functional modulation with increasing model complexity, demonstrating functional validation of the model. Image Credit: Axol Bioscience Ltd
Blinded in vitro compound screening using electrophysiological endpoints
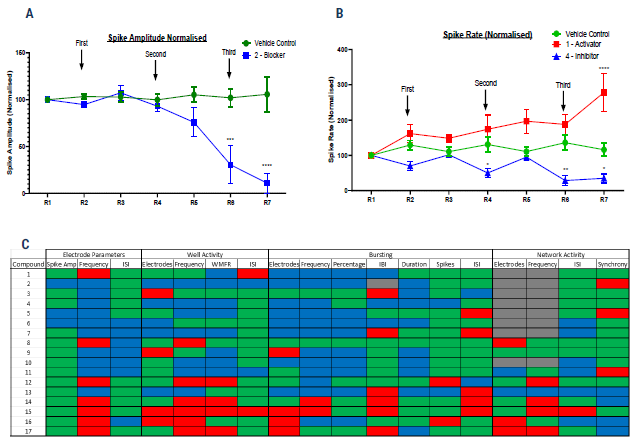
Figure 3. Blinded compound screening on a cortical tri-culture axoModel, with electrophysiological endpoints measured on the Axion Maestro MEA system. For more details on Axol Bioscience’s tri-culture protocol timeline, please see the QR code at the bottom. (A) Example spike amplitude data for vehicle control and Compound 2 (a “Blocker”) (B) Example spike rate data for vehicle control, Compound 1 (a “Hyperpolarizing Activator”) and Compound 4 (an “Inhibitor”). Statistical significance was assessed by two-way ANOVA. (C) Heatmap of the monitored parameters on the Axion MEA for the blinded compound list. Seventeen compounds were tested in total, with nine novel compounds of undefined action, two anonymized proprietary compounds, designated Compound A and Compound B, and six compounds of known action. Each parameter was color-coded GREEN for Unchanged, RED for Increased and BLUE for Decreased compared to the vehicle control.
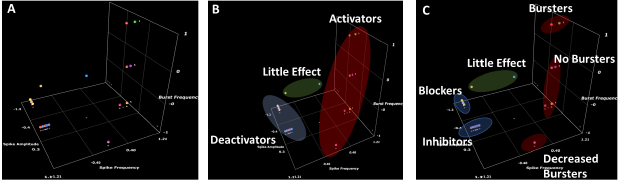
Figure 4. 3D scatter plots of measured MEA parameters for blinded compounds and vehicle control. (A) Unclustered data (B) Data clustered by highest level groupings (Little Effect, Deactivators and Activators) (C) Data clustered by sub-groupings (Little Effect, Blockers, Inhibitors, Increased Bursters, Unchanged Bursters and Decreased Bursters). Increased Bursters were identified as potentially proconvulsant, Unchanged Bursters are potentially non pro-convulsant and Decreased Bursters are hyperpolarizing. Created using the software available at https://miabellaai.net/. Image Credit: Axol Bioscience Ltd
Unblinding of compounds
| Compound |
Group |
Subgroup |
| Number |
Name |
Model
classification |
Unblinded/
literature
classification |
Model
classification |
Unblinded/
literature
classification |
| 1 |
NS 309 |
Activator |
Activator |
Activator – Decreased Bursting |
Activator – Decreased Bursting |
| 2 |
Compound A |
De-activator |
De-activator |
De-activator- Blocker |
De-activator - Inhibitor |
| 3 |
Nifedipine |
Little Effect |
Little Effect |
Little Effect |
Little Effect |
| 4 |
Compound B |
De-activator |
De-activator |
De-activator- Inhibitor |
De-activator- Inhibitor |
| 5 |
Lamotrigine |
De-activator |
De-activator |
De-activator- Blocker |
De-activator- Blocker |
| 14 |
4-AP |
Activator |
Activator |
Activator – Increased Bursting |
Activator – Increased Bursting |
| 16 |
Levetiracetam |
Activator |
Activator |
Activator – Unchanged Bursting |
Activator – Unchanged Bursting |
| 17 |
Lithium Carbonate |
Activator |
Activator |
Activator – Unchanged Bursting |
Activator – Unchanged Bursting |
Figure 5. Table of unblinded compounds showing almost perfect fidelity between model-based predicted action and actual action. After unblinding, the actual reported action of the compound was compared to the predicted effect using the Axion Maestro MEA system. Results demonstrated was alignment of the predicted and actual Group in 8 out of 8 compounds, with correct prediction of the Subgroup in 7 out of 8 compounds. These results were deemed by the client to be a valuable neurological compound screening test system, with future applications in drug discovery and neurotoxicity screening. Image Credit: Axol Bioscience Ltd
Conclusion
In this study, a human iPSC-derived cortical tri-culture axoModel was utilized to correctly distinguish the effects of 8 compounds, with alignment of 8 out of 8 Groups and 7 out of 8 Subgroups.
Using human iPSC-derived cells in advanced in vitro platforms improves the production of human-relevant experimental models by better exemplifying the complex in vivo interactions that occur in the human cortex compared with equivalent heterologous cell lines and animal models.
While these tests were conducted with cells from the same healthy control donor, it is feasible to include lines taken from numerous donors and patients with NDDs, including Alzheimer’s, to increase complexity and develop in vitro models specific to certain diseases for developing new drugs.
Download the Poster Version
References and further reading
- Feigin V.L., Vos T., Nichols E., O Owolabi M., Carroll W.M., Dichgans M., Deuschl G., Parmar P., Brainin M., Murray C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2019;19:255–265. doi: 10.1016/S1474-4422(19)30411-9.
- Richard J Weaver, Jean-Pierre Valentin, Today’s Challenges to De-Risk and Predict Drug Safety in Human “Mind-the-Gap”, Toxicological Sciences, Volume 167, Issue 2, February 2019, Pages 307– 321, https://doi.org/10.1093/toxsci/kfy270
About AXOL Bioscience
The first choice for high-quality, functionally relevant iPSC-derived cells.
With over a decade of experience, we’ve developed the manufacturing capabilities to produce high-quality, functional iPSC-derived cells with excellent consistency.
Your research can benefit from our quality-focused approach, with our catalog of robust, highly relevant iPSC-derived neurons and cardiomyocytes developed at our ISO 9001:2015-accredited production facility.
Our leading neuronal cell types include: cortical excitatory neurons, striatal neurons, cortical inhibitory interneurons, microglia, astrocytes, sensory neurons and motor neurons. We also provide high-quality atrial cardiomyocytes and ventricular cardiomyocytes, as well as made-to-order myotubes.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.