Poliovirus is a member of the family of picornavirus. Picornaviruses are small, about 300 angstroms in diameter, and are comprised of an icosahedral protein coat and a single-stranded positive sense RNA genome.
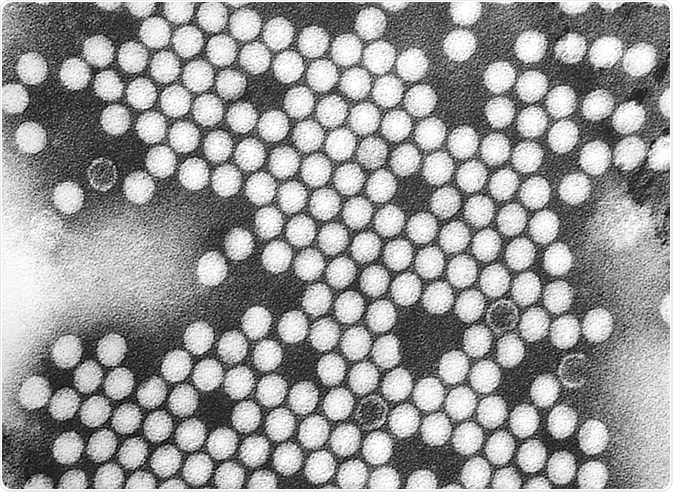 Electron micrograph of polovirus virions. Credit: Everett Historical/Shutterstock.com
Electron micrograph of polovirus virions. Credit: Everett Historical/Shutterstock.com
Poliovirus infection occurs by the fecal-oral route. The host ingests the virus, which replicates in the alimentary tract. The virus is then shed in the feces. Most polio infections are asymptomatic. In about 5 percent of cases, the virus replicates in other tissues. Paralytic poliomyelitis occurs in less than 1 percent of cases.
The viral proteins of poliovirus are:
- 3D(pol): an RNA dependent RNA polymerase
- 2A(pro) 3C(pro)/3CD(pro): proteases that cleave the viral polypeptide
- VPg (3B): binds viral RNA and is required for synthesis of RNA
- 2BC, 2B,2C, 3AB, 3A, 3B: protein complex needed for viral replication
- VP0: cleaved into VP1 and VP3, and VP2 and VP4, the proteins of the viral capsid
Molecular structure
The poliovirus capsid contains 60 copies each of the four viral polypeptides VP1, VP2, VP3, and VP4. The arrangement of proteins in the capsid creates icosahedral symmetry. The virion surface is covered with star-shaped mesas at its fivefold axes surrounded by deep canyons and three-bladed propellers.
These are situated at threefold axes separated by saddle depressions straddling twofold axes. Capsid proteins VP1, VP2, and VP3 all have an eight-stranded β-barrel fold, but have different shaped loops on their N- and C-terminal extensions.
Entry into cells
Poliovirus infection begins with the virus binds to the receptor CD155 on the host cell surface. CD155 is an immunoglobulin-like receptor also known as poliovirus receptor (PVR). The PVR polypeptide has an N-terminal sequence of three extracellular immunoglobulin-like domains, a transmembrane domain, and a cytoplasmic tail. The cellular function of PVR is unknown, but it is thought to play a role in cell adhesion and recognition.
Upon binding, an irreversible conformational change occurs to the viral particle. The altered form of the viral particle is known as the A particle. When bound to cells at 37°C in vitro, certain changes have been observed.
The sedimentation coefficient of the particle is 135S, compared to 160S for particles in their native conformation. The 135S particles are more sensitive to detergents and lack VP4. The N terminus of VP1 in the native particle is on the inside, but in the 135S particle, VP1 has been translocated to the surface, rendering the capsid hydrophobic.
Structural studies of the 135S particle showed that the N terminus of VP1 exits the capsid through an opening in the interface between VP1 and VP3 near the base of a canyon that surrounds the fivefold axis of the molecule. The externalized N terminus is located near the tips of propeller-like extensions near the threefold axis rather than the fivefold axes.
Particle A is believed to be a necessary intermediate to entry of the poliovirus into cells. It is thought that the N terminus of VP1 forms an amphipathic helix that inserts itself into the cell membrane, creating a pore through which the viral RNA passes from the capsid into the cytosol. Another possible mechanism by which the virus enters the cell is believed to be receptor-mediated endocytosis, after which the viral RNA is released into the cellular cytoplasm.
Further Reading
Last Updated: Feb 26, 2019