Sep 27 2010
The National Institutes of Health has awarded a prestigious EUREKA award to Robert Woods, a professor at the University of Georgia Complex Carbohydrate Research Center, to develop a new method for understanding how antibodies interact with large molecules known as glycans that are a major component of all cell surfaces.
Glycans, which are essentially big sugar molecules, stimulate the body’s immune system to produce antibodies that combat disease when they encounter proteins from bacterial and viral intruders. Scientists are searching for better molecular tools to help them characterize disease-related glycans, as this could be an important step in diagnosing and developing therapeutics for a host of illnesses ranging from cancer to diabetes.
These complex twisty and branching carbohydrate molecules have stymied biotechnology researchers for years. Their sheer number, as well as the rapidly changing shapes of the structures, has made it impossible for scientists to precisely define the glycan that a protein is interacting with. Even with the assistance of high-power computing and established analytical techniques, such as nuclear magnetic resonance (NMR) and X-ray crystallography, characterizing the way glycans interact with other proteins—interactions that are often associated with bacterial and viral infections—has been far from routine.
Recognizing the scientific rewards for biology and medicine that would come from solving this problem, the NIH has awarded a EUREKA award to Woods to develop a new method for characterizing diagnostic antibody specificity. The EUREKA award, given to support “Exceptional, Unconventional Research Enabling Knowledge Acceleration,” will provide Woods with approximately $200,000 per year for four years.
Woods is a professor in the department of biochemistry and molecular biology, in the Franklin College of Arts and Sciences, and professor and chair of Computational Glycosciences, School of Chemistry, National University of Ireland, Galway.
.jpg) |
| Figure 1: Antibodies are often used to diagnose and develop treatments for disease; their development is the most rapidly growing area of the pharmaceutical industry. Shown is a computer-simulated 3D structure for an antibody that can recognize specific types of cancers on the basis of the glycans (complex carbohydrates, green structure) present on the cancer cell surface. |
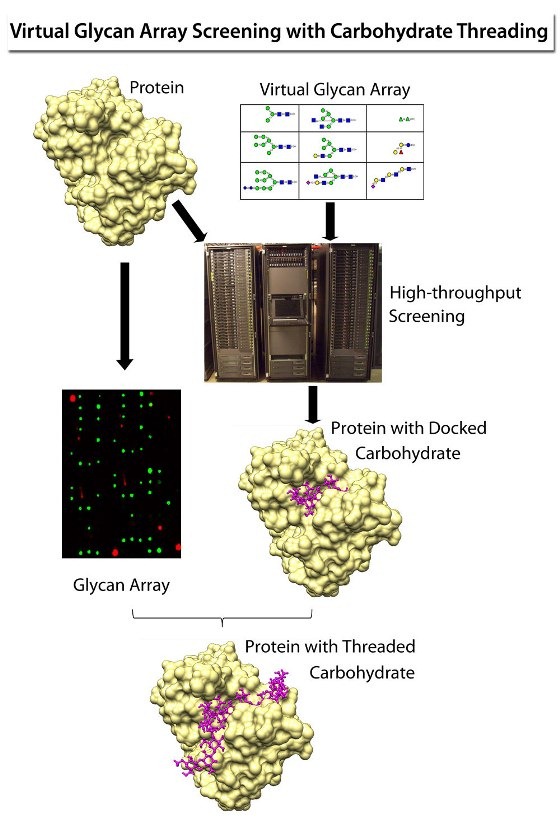 |
| Figure 2: In a combination of methods called “carbohydrate threading,” data obtained from two high-throughput technologies – virtual glycan array screening and experimental glycan array screening – are combined to produce the 3D structure of a protein, such as an antibody, in combination with a large carbohydrate. Producing a structure of this complexity would be difficult using either X-ray crystallography or NMR spectroscopy. |
The problem, according to Woods, has been that pertinent data from traditional analytical methods are extremely difficult to generate. Established ways of generating 3D molecular structures are not necessarily applicable to complex carbohydrates, he explained, as the large carbohydrate molecules, unlike proteins, are floppy and often “too big” or too heterogeneous for these methods. Woods said that with current methods of defining the 3D structures of carbohydrate-protein complexes, “determining the 3D structure for a potential diagnostic agent becomes a Ph.D. thesis.”
During the last 10 years, he said, the technology to identify disease-related glycans has evolved rapidly, principally due to advances in mass spectrometry and glycan array screening, two technologies that speedily analyze millions of samples. However, Woods said that as libraries of carbohydrate structures have grown, “among the things we’ve discovered is that antibodies against carbohydrates are not as specific as we had thought. That can lead to false positives or unanticipated cross-reactions—results that diagnostically and therapeutically are very bad. We can predict 3D structures, but without some corroborating data from the laboratory, people should be cautious before putting a lot of trust in it.”
Now, said Woods, combining two disparate high-throughput technologies—glycan array screening and computer modeling—provides a new approach to generating detailed atomic-level structures that provide insight into the origin of protein-glycan specificity.
Woods said the field of computational glycoscience holds promise for making structural information about such interactions widely accessible to the biotechnology industry. “This insight can guide the engineering of enhanced antibody specificity or assist in the development of antibodies as therapeutic agents,” he said. “We should be able to re-engineer the antibodies to be more specific and more effective as therapeutics and diagnostics.”