
An interview with Ed Moriarty, discussing how Bose are involved in the development of advanced testing equipment for medical devices.
Bose is best known as an audio company. How did Bose come to be involved in the testing equipment business and how does better audio link to advanced testing equipment?
Bose has been committed to electromagnetic technology and research since its founding close to 50 years ago. In the audio field, it’s well known that reproducing sound with lifelike detail requires extraordinary control of a loudspeaker’s motion.
Image Credit: ALEXEY FILATOV/Shutterstock.com
Several years ago, while working on an experimental loudspeaker, Bose engineers developed a new kind of specialized electromagnetic linear motor with a patented, friction-free durable design.
Additional research revealed that the technology in this new linear motor could be used to create test instruments with exceptional fidelity and precision. As a result, the Bose ElectroForce Systems Group has met a significant need in the marketplace for clean, high-frequency electrodynamic test systems.
Over the last 15 years, we have developed a full line of materials testing and durability simulation instruments to research institutions, universities, medical device companies and engineering organizations worldwide.
Please can you introduce the Bose® 3200 Series III test system? What test applications is this well-suited to?
The new Bose® 3200 Series III test system offers research and development engineers improved accuracy, performance and ease-of-use for materials testing and medical device development.
The Series III test system utilizes advanced stroke measurement technologies to increase the lower range of reliable displacement data by a factor of 10 compared to previous Bose offerings.
With measurement resolution of one nanometer and accuracies in the range of microns, the Series III system provides highly useful low-amplitude displacement information to designers, helping them to better understand small-scale behaviors that impact material and component properties.
This advanced electrodynamic test system also delivers fatigue testing response up to 300 Hz and Dynamic Mechanical Analysis (DMA) testing up to 200 Hz, making it faster and more efficient for many biomedical and engineered materials test applications, including biomaterials, tissues, elastomers, microelectronics, polymers and composites.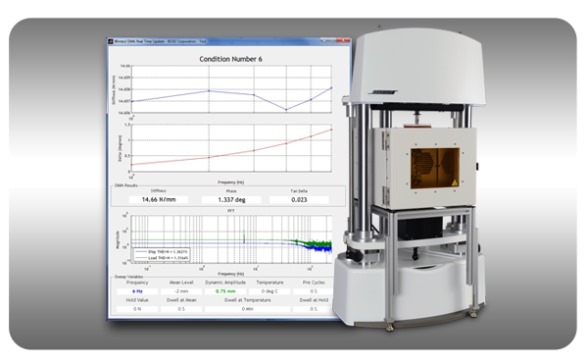
Bose DMA Test Instrument with Application Software
What are the main reasons behind the need for testing to become more accurate and rapid?
Bose test instruments help customers design better products and bring them to market faster. Low-amplitude displacement testing accuracy is an increasing priority for the development of biomaterials and new engineered materials.
Engineers increasingly rely on material property data to design advanced products for applications such as microelectronics, low-amplitude material fatigue characterization, small bone or tissue property measurements, and DMA testing of elastomers and tissues. The Bose® 3200 Series III test instrument achieves unprecedented measurement resolution and accuracy for electrodynamic test systems, which is critical for this type of testing.
Getting reliable products to market quickly is a key driver for all types of companies. Higher frequency fatigue testing of materials and components allows the testing process to move faster, ultimately shortening time to market.
What are the main hurdles involved in developing a test system that is both highly accurate and dynamic?
A high-performance load and motion control system is critical for these types of performance needs. Proprietary linear motors developed by Bose research engineers are at the heart of ElectroForce test instruments, and they provide exceptional performance and energy efficiency without compromising either.
The Bose® ElectroForce linear motor provides an attractive alternative to traditional motion and force control because of its simple, durable, moving-magnet design. The motor utilizes a friction-free, flexure suspension in order to achieve exceptional fidelity and precision. With its inherent low-noise operation and clean, environmentally friendly design, the Bose® 3200 Series III test instrument is well-suited for many low-force test applications, while also providing excellent dynamic displacement at accelerated test frequencies.
For high-frequency test systems, acceleration-induced force errors are a key issue to overcome. The new Bose system is equipped with a new proprietary acceleration compensation algorithm to correct these types of errors. The compensation system ensures higher accuracy force measurement while also providing improved control at higher frequencies for a variety of test situations.
What impact do you think the Bose® 3200 Series III system will have on the testing market?
There are many challenges that researchers and development engineers face in creating the next generation of materials, devices and products. More than ever, our customers need to have the highest confidence in the data they’re using throughout the development process.
The Bose® 3200 Series III test instrument delivers both low-level measurement precision and high-frequency dynamic response in one test system. The range of performance and versatility is unprecedented, allowing researchers to perform a wide range of advanced property characterization for a variety of test samples.
We’re confident that customers will find a wide variety of new applications for our Series III test system, many targeted at new tests to better understand material and component properties.
It’s an exciting time in materials research and biomedical development, and we’re excited to play a role in helping customers increase their confidence in creating the next generation of materials, devices and machines.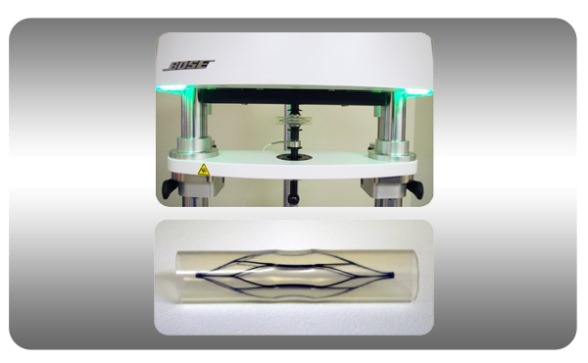
Characterization of Load and Deformation Properties for a Cardiovascular Medical Device
What do you think the future holds for biomedical testing equipment and how does Bose plan to contribute to this?
We are seeing increasing interest from our customers to help them with their development projects related to new materials and devices. Advances are occurring rapidly in a variety of biomedical applications. Many of these new products directly improve the quality of life for patients or extend their life.
The Bose ElectroForce Systems Group is committed to helping manufacturers and researchers design better products – from cardiovascular devices to engineered materials and biomaterials – and get them to market faster.
We work closely with customers to first understand their requirements, and then create a tailored solution so they can better reach their goals.
Our heritage and commitment to electromagnetic technology and research enables us to successfully meet the needs of the vibrant and growing biomedical materials and device market.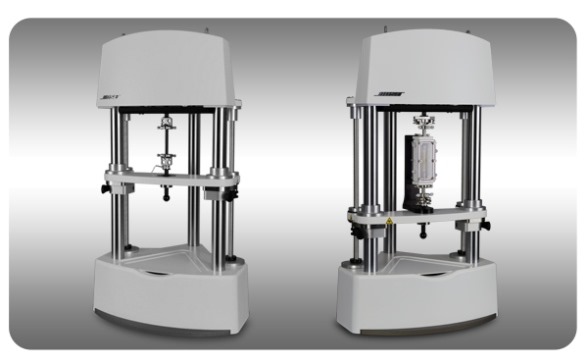
Bose Series III Configurations for Materials Testing (left) and Tissue Engineering (right)
Where can readers find more information?
ElectroForce Mechanical Tesing Instruments
About Ed Moriarty
 Ed Moriarty has been the General Manager of the ElectroForce Systems Group of Bose Corporation since 2004. ElectroForce was the first non-audio division within Bose.
Ed Moriarty has been the General Manager of the ElectroForce Systems Group of Bose Corporation since 2004. ElectroForce was the first non-audio division within Bose.
Prior to the ElectroForce assignment, Ed was the Director of the North American business unit within the Automotive Systems Division of Bose.
Ed has a BS in Engineering and Economics from Union College, Schenectady, NY and an MBA from Rivier College, Nashua, NH.