HbA1c and ß-HB analyzers enable rapid assessment of diabetes and related conditions
EKF Diagnostics, the global in vitro diagnostics business, will be exhibiting two newly introduced point-of-care (POC) testing analyzers for rapid assessment of diabetes and related conditions. The Quo-Lab glycated haemoglobin (HbA1c) analyzer and STAT-Site M ß-HB will both be demonstrated on the Welsh Assembly Government Stand Z1D30 in Zabeel Hall 1, Arab Health, Dubai International Convention & Exhibition Centre, 27 - 30 January 2014.
Although only launched in July 2012, close to 1,300 Quo-Lab HbA1c analyzers were sold globally in 2013, 60 of which are now installed in Lebanon for near-patient measurement and monitoring of diabetes. HbA1c monitoring is increasingly used in the detection and management of diabetes and the Quo-Lab provides highly accurate, affordable and easy-to-use technology, which uses just 4 µL of venous or finger prick blood to deliver lab-accurate results within four minutes.
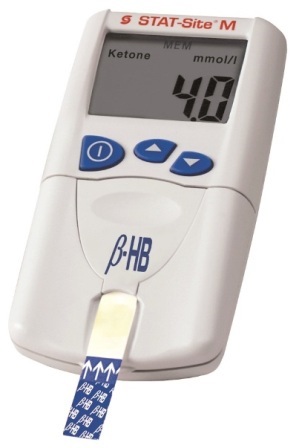
The STAT-Site M ß-HB analyzer from EKF Diagnostics. Image Credit: EKF Diagnostics
Small and lightweight, Quo-Lab is designed specifically to meet the needs of clinics and laboratories in emerging economies, where diabetes is an increasingly large public health issue, and is ideal for GP surgeries, diabetes clinics and laboratories.
EKF’s new STAT-Site M ß-HB will also be available for demonstrations. This new quantitative strip-based test for ß-Hydroxybutyrate (ß-HB) is CE marked and provides a quick and easy assessment of diabetic ketoacidosis (DKA), delivering results in less than 80 seconds.
Using the STAT-Site M ß-HB, the presence and degree of ketosis can be rapidly assessed at the point-of-care by accurately measuring blood levels of ß-HB, which accounts for 78% of the three ketone bodies, from just 10 µL of serum or plasma. This provides useful information to provide quality care when monitoring DKA in newly diagnosed patients, whilst also reducing time and costs in an emergency room setting.