Advanced Cell Diagnostics Inc. (ACD), a leader in the field of molecular pathology and developer of cell and tissue-based analysis tools, has announced that its RNAscope® RNA in situ hybridization technology has reached two major milestones. In just three years, over 100 peer-reviewed papers featuring the technology have been published, and with the significant increase in use of RNAscope, ACD has now built a library of over 4000 target probes for numerous species. Probes are designed to order in under two weeks, and in just six months the library has grown by over 1500, reflecting the wide interest in ACD’s breakthrough technology.
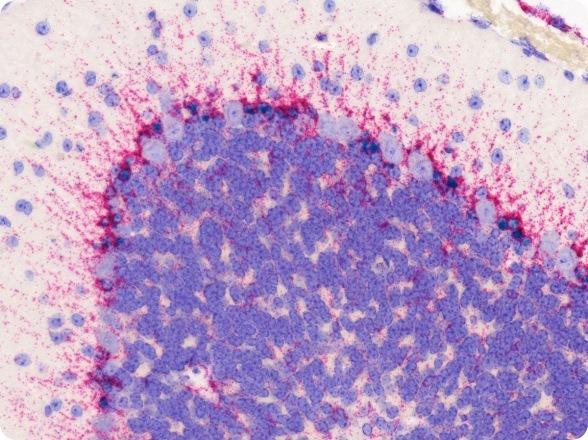
Gli1 and APOE mRNA expression in mouse brain FFPE tissue using RNAscope® 2-plex Chromogenic Reagent Kit
RNAscope uniquely has the sensitivity to enable researchers to detect in situ single RNA molecules and provide quantitative analysis of gene expression at a single cell level. In addition, the technology provides morphological context by showing spatial and cell-specific expression while preserving tissue architecture. RNAscope’s ability to unlock the full potential of RNA biomarkers, together with its highly reproducible and easy-to-use technology, has resulted in an average of over 6 papers a month published so far in 2014 - a rate that is doubling every year. Now in wide use throughout academia and industry, the papers range from basic research in developmental biology, neuroscience and stem cells to clinical research such as cancer biomarkers, infectious diseases and ophthalmology, in respected journals such as Nature, Science, Cell, PLoS One, PNAS and Clinical Cancer Research.
Localizing and quantifying RNA sequences in the context of cells and tissues is a fundamental approach in molecular biology. RNAscope makes it accessible to researchers of any level of experience, as Alexey Pronin, PhD of the University of Miami School of Medicine, who recently published in PLoS One, explained. “Even though I had no previous experience of in situ hybridization, the RNAscope assay was easy to perform and worked first time, allowing us to confirm the expression of three different genes in the mouse eye that we had previously identified via transcriptomics. Importantly, the multiplex assay showed that two of the genes are expressed in two separate cell layers of the eye blood vessels – information that would be hard to get using other technologies.”
“Publications from our customers are particularly exciting, as it shows the growing validation and adoption of our technologies at the forefront of scientific research”, said Xiao-Jun Ma, ACD’s CSO. “And with our probe catalog growing by 240% in the last year, targeting more than 4,000 genes in many species, it’s a real testament to the demand for our technology, our fast probe development times and the scalability of our platform. Together, these two milestones are a comprehensive validation of the effectiveness of RNAscope technology. In this age of single-cell transcriptomics, RNA in situ hybridization will prove to be indispensable in the effort to characterize the many newly discovered genes, especially the vast repertoire of noncoding RNA genes. We believe that the specific benefits of RNAscope technology will undoubtedly accelerate the translation of genomic discoveries to clinical medicine including new therapeutics and diagnostics.”
For more information about the published research on RNAscope, please visit https://acdbio.com/
About Advanced Cell Diagnostics, Inc. (ACD)
Advanced Cell Diagnostics, Inc. (ACD) is a leader in the emerging field of molecular pathology, developing cell and tissue-based research tools for all areas of biomedical research, and diagnostic tests for personalized medicine. The company’s products and services are based on its proprietary RNAscope® technology, the most sensitive method available for RNA in situ hybridization, and the first quantitative and fully automated platform enabling multiplex fluorescent and chromogenic RNA biomarker analysis. ACD partners with pharmaceutical and biotechnology companies to validate biomarkers for targeted therapeutic development in cancer and other diseases. These partnerships provide the foundation for ACD to develop companion diagnostic tests in conjunction with partners’ targeted therapeutics. ACD also pursues internal programs to develop proprietary diagnostic tests in cancer management. Learn more about ACD and RNAscope® technology at www.acdbio.com.