Flagship Biosciences and Advanced Cell Diagnostics collaborate to develop quantitative RNAscope® assays for pharmaceutical clinical trials in the CLIA environment.
Flagship Biosciences, the leading service provider of tissue image analysis solutions, announced today the utilization of its wet assay development and image analysis quantification capabilities for RNAscope® RNA in situ hybridization (ISH) from Advanced Cell Diagnostics, Inc. (ACD), a technology and market leader in the field of molecular pathology and developer of cell and tissue-based analysis tools. The two companies will work to optimize quantitative image analysis of in situ hybridization in tissue samples in Flagship’s CLIA laboratory.
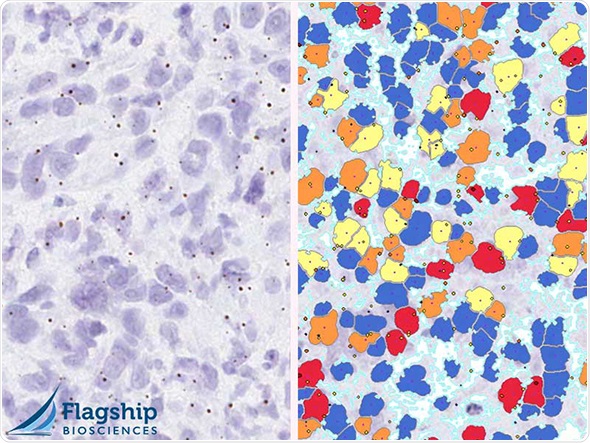
The increasing use of RNA ISH in companion diagnostics for drug development and CDx strategies require multidisciplinary expertise in pathology, assay development and image analysis in a CLIA laboratory environment. Flagship has leveraged ACD’s industry-leading in situ hybridization assay platform to support its clients ongoing CLIA based clinical trials to enable the utilization of RNAscope technology in CDx trials.
Dr. Joseph Krueger, Chief Science Officer for Flagship Biosciences said:
Flagship focusses on delivering unique fit-for-purpose quantitative pathology results, with advanced experience in both IHC and ISH assay development and custom image analysis. Over the last five years, our customers have benefited from our ability to focus our resources on unique scientific measurements using image analysis tools, which are able to overcome the obstacles barring clinical utility. By partnering with ACD, we are able to provide the state-of-the art in situ hybridization approaches, with quantitative endpoints suitable for a companion diagnostic strategy
Dr. Xiao-Jun Ma, Chief Scientific Officer of ACD said:
One critical feature of the RNAscope assay is its ability to detect individual RNA molecules within the context of tissue morphology. This enables a quantitative expression readout at individual cell level, which opens the door to a higher level of precision biomarker analysis. We are very pleased to work with Flagship to fully leverage this advantage, which is particularly important for the development of companion diagnostics