Scientists from Cardiff and Swansea Universities are combining the principles of the butterfly effect and computer simulation to explore new ways of predicting and controlling the beginnings of heart disease.
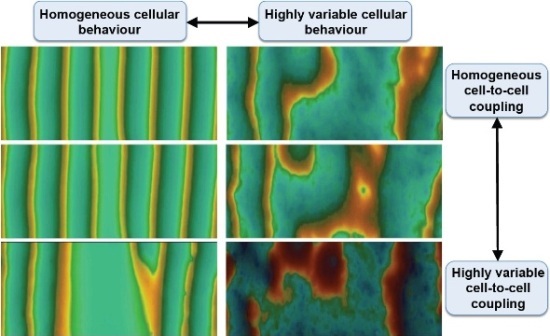
Each panel depicts the organization of signals within a computational array representing approximately 6000 individual cells. As events within individual cells become more variable compared to their neighbours (right versus left) the organization of signals across the entire array becomes much more susceptible to breakdown in response to changes in cell-to-cell interaction (top to bottom). This figures illustrates the origins of large scale desynchronization of cell networks. This type of unravelling in heart cell networks is a key driver in the onset and progression of heart disease.
The harnessing of computer models to visualise communication between heart cells is paving the way for screenings to identify characteristic flashpoints associated with degenerative changes in the heart. These insights, researchers hope, will enable them to anticipate disease years before symptoms occur, allowing for a timely intervention to halt and reverse its progress.
Published in the Annals of Biomedical Engineering journal, researchers describe being able to disrupt a network of healthy heart cells by using channel-blocking drugs, or chemicals that interfere with intracellular calcium signals, to create a ‘wave’ of poor communication among cells. Importantly, these experiments also gave clues as to how order and healthy communication could be restored to a network of disrupted heart cells using the same approach.
Dr Christopher George, a molecular cardiologist from Cardiff University, describes healthy hearts as being totally dependent on the synchronization of huge networks of cells working together for a common purpose; while disease, he says, can be defined as a loss of synchronicity whereby communication between individual cells is either very poor or lost altogether.
“Much like a murmuration of starlings in flight, the synchronization and behaviour of thousands of birds gives rise to complex patterns that also have an amazing simplicity to them,” explains Dr George. “If a few birds don’t conform to these patterns, it wouldn’t make a great deal of difference – the overall collective behaviour would remain.
“As in the butterfly effect, small changes can lead to drastic changes: if, over time, more birds become uncoupled from the dominant flight pattern, either a new pattern of communication would emerge, or the entire network would collapse into disarray.
“Our research shows that this is what happens to human cells in heart disease: the well-ordered behaviour of coupled cells unravels and the synchronization is lost. Along this route though, there are points at which is possible to halt, or reverse, this de-synchronization. We call these the ‘crisis points’.”
Dr Dimitris Parthimos, a mathematician based in Cardiff University’s Wales Heart Research Institute, developed the mathematical models that were used to perform the computer simulations. He said:
We are shedding light on a key driver in the development and progress of heart disease that causes rhythm disturbances and abnormal contraction.
In knowing how to decode the patterns of cell communication, we can now begin using this information to design new ways of modulating cell behaviour, to delay the onset of heart disease, and ultimately develop methods to reverse the process if disease is already established. Since all organs within the human body depend on communication networks built by cell-to-cell coupling, this work has implications for other diseases from cancer and neurodegeneration to diabetes.
According to the paper, the behaviour of cell networks, called ‘arrays’, conforms to a branch of mathematics known as non-linear dynamics – also known as ‘the butterfly effect’ - of which there are two vital features: firstly, if a sufficiently detailed picture of cell signalling can be constructed then the outcome of cell behaviour, in response to a ‘crisis point’, can be predicted. Secondly, if scientists understand the nature of these crisis points, which trigger the progression of disease, it should be possible to reverse it.
Dr George continued:
Everything we are and everything that we do results from well-orchestrated biological signals or - ‘switches’ - that use very few components. What we’re trying to do now is to identify the ‘on’ and ‘off’ switches that turn health into disease, so as to understand the very early events that cause disease. This will help make it possible to intervene early, even before symptoms appear. We can then look to design ‘switches’ that turn disease back into health.
Professor Jeremy Pearson, Associate Medical Director at the British Heart Foundation, which helped fund the research, said:
Mild heart rhythm disorders, or arrhythmias, are common. If left untreated they may lead to more serious problems.
This new approach of using mathematical modelling to simulate how arrhythmic waves of contraction occur in the heart could lead to the development of ways to block arrhythmias more effectively in patients.
With over a million people in the UK living with an arrhythmia, advances in treatment that can improve and even save lives are urgently needed.
The research team envisages that developments in imaging technologies will in the near future help to construct detailed 3D maps of entire human hearts, giving them an even more accurate picture of how heart cells communicate with each other, and the tell-tale signs of when things go wrong.