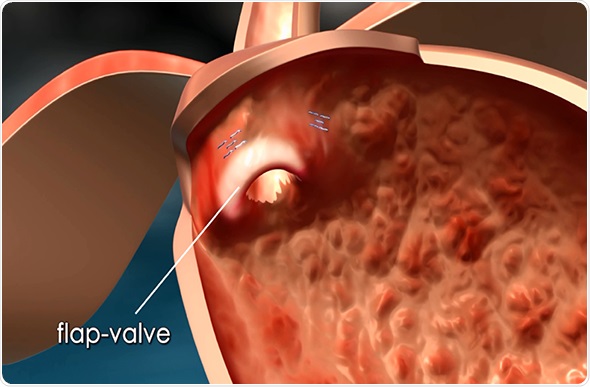
Currently, the leading laparoscopic method for the long-term treatment of GERD is the Nissen fundoplication — a surgical procedure in which the fundus (the upper part of the stomach) is wrapped (plicated) around the lower esophageal sphincter and typically sewn into place. This procedure is intended to reinforce the lower esophageal sphincter (LES) to help prevent stomach acids from backing into the esophagus and causing irritation.
The main drawback of this method is that the procedure requires incisions, making it likely that patients will experience additional discomfort and have a longer recovery time. This is both inconvenient for patients and more costly for healthcare institutions.
Why is there an existing gap in GERD treatment between drug therapy and invasive laparoscopic procedures?
To control reflux symptoms, many people take acid-controlling medications such as Proton Pump Inhibitors, or PPIs. The FDA, however, has issued warnings over the frequent or long-term continued use of PPIs, citing concerns that overuse can lead to osteoporosis, interference with nutrient absorption or other digestive complications.
However, many people choose not to undergo surgical fundoplication procedures, because they feel it is too invasive and rather stick with medication. For people who fall within this treatment gap, MUSE offers a more patient-friendly, incisionless alternative to laparoscopic surgery, for the long-term treatment of GERD.
Please can you give a brief overview of the Medigus Ultrasonic Surgical Endostapler, or MUSE?
The MUSE system is an intuitive, endoscopic platform for the long-term, possibly permanent, treatment of GERD. It is equipped with the latest technological advancements in microvisualization, ultrasound and surgical stapling, thereby allowing a single physician or surgeon to perform anterior partial fundoplication more easily than with leading laparoscopic methods.
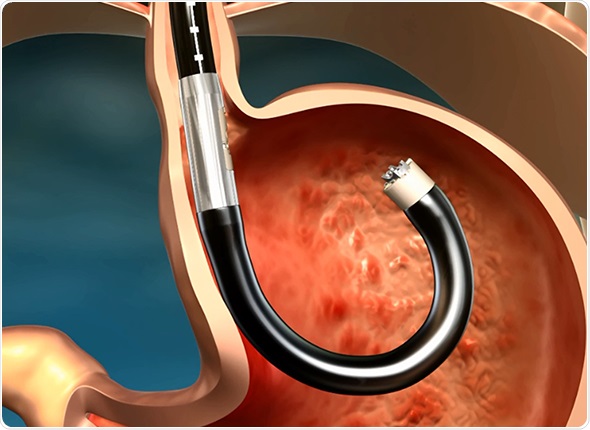
Its platform consists of a single use flexible surgical endostapler, equipped with a proprietary miniature camera, an ultrasonic sight and a range finder, and includes a handle with controls, an 80cm flexible shaft, a 5cm rigid section holding a cartridge with 5 standard 4.8mm titanium surgical staples, a ratchet controlled one-way articulating section, and a distal tip.
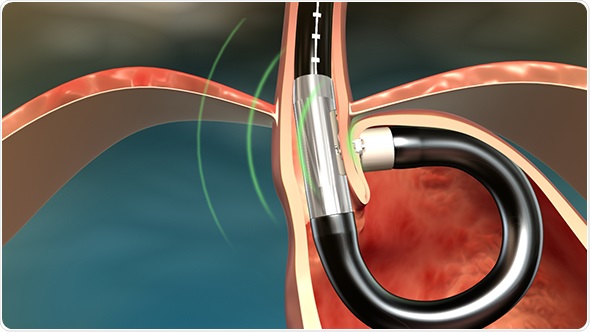
The MUSE endostapler is progressed into the stomach through an overtube and retroflexed under direct video guidance. After identifying an ideal stapling location, the stapler is gently pulled back to place the staple cartridge in the lower esophagus approximately 3cm above the gastroesophageal junction. The procedure typically delivers about five staples to effectively reinforce the LES.
The device’s video camera offers direct visualization during insertion and staple site selection, and ultrasound to determine when a proper stapling gap is achieved.
At what stage of development is MUSE at?
The MUSE system is FDA cleared and CE marked for the treatment of GERD and is already in use by leading gastroenterologists and GI surgeons throughout the United States and Europe.
Renowned gastroenterologist, Professor Pier Alberto Testoni, Director of Gastroenterology and Digestive Endoscopy IRCCS at San Raffaele Hospital in Milan, recently completed three MUSE procedures. What feedback did he give?
He was happy to report that all patients were released from the hospital within two days following their procedure, and have reported no complications.
In what ways do you think MUSE will improve GERD-related quality of life for patients?
Those who suffer from persistent GERD may find that, following treatment with MUSE, they have significantly reduced, or perhaps altogether eliminated the use of PPIs, which could contribute to an improvement in GERD-related quality of life.
In a recent study, six weeks following treatment with the MUSE system, 92% of patients had stopped the use of daily GERD medications and had normalized quality of life scores, while 69% had eliminated use of GERD medications altogether. Average esophageal acid content was also significantly reduced following the procedure.
After 5 years, over 90% of study participants maintained their normalized quality of life measures and approximately 77% had ceased taking daily GERD medications.
Without having to adhere to potentially restrictive dietary or medication regimens, people may be able to enjoy more physical activities, greater freedom in food choices or even simply feel more comfortable without suffering the troublesome symptoms of reflux.
How do you hope to improve MUSE going forwards?
Our focus is currently on rolling out across the United States and Europe.
What do you think the future holds for GERD treatments and how do Medigus plan to add to it?
I think the GERD market will become stratified and offer the patient several less invasive options on the path between PPT and LNF. Medigus plans to focus on an existing and effective procedure (lap fundoplication) only in a new less invasive approach (trans-orifice).
Where can readers find more information?
You can find more information about the MUSE system from www.medigus.com. The site features useful information for patients as well as physicians.
About Chris Rowland
Chris is CEO of Medigus and serves as a member of the board of directors.
Earlier, Chris was President and CEO of NeoTract, Inc. From 2006 to 2009 he served as President Americas and was a member of the Executive Committee for Given Imaging (NASDAQ: GIVN – www.givenimaging.com).
Prior to his position at Given Imaging, Chris worked for the Boston Scientific  Corporation (www.bsci.com) for seventeen years in many divisions in positions of increasing responsibility from Sales, Product Development, Marketing, Business Development and General Management. Chris has also held a position at Xerox Corporation.
Corporation (www.bsci.com) for seventeen years in many divisions in positions of increasing responsibility from Sales, Product Development, Marketing, Business Development and General Management. Chris has also held a position at Xerox Corporation.
During his career, Chris has received over 25 Patents for a variety of minimally invasive medical devices. Chris has a B. Sc. in Marketing from Southern Illinois University and has completed the Executive Leadership Program at Harvard Business School and the Executive Management Program at Columbia Business School.